
Head of the group: Candidate of chemical sciences O.V. Golovchenko
Publications:
SYNTHESIS OF NEW PHOSPHONOPEPTIDOMIMETICS /
СИНТЕЗ НОВЫХ ФОСФОНОПЕПТИДОМИМЕТИКОВ
ER Abdurakhmanova, OI Lukashuk, AV Golovchenko, VS Brovarets
Keywords: 4-phosphorylated 5-amino-1,3-oxazoles, phosphonopeptidomimetics, aminoalcohols
Keywords: 4-phosphorylated 5-amino-1,3-oxazoles, phosphonopeptidomics, aminoalcohols.
One of the promising methods of obtaining of phosphorylated peptidomimetics is the acid cleavage of the 5-amino-1,3-oxazole cycle. For the purpose of introducing into the phosphonopeptide chain of the ethanol moiety, we obtained {5 - [(2-hydroxyethyl) methylamino] -2-phenyl-1,3-oxazol-4-yl} phosphonic acid (III) diethyl ether from diethyl ether 1- benzoylamino-2,2,2-trichloroethylphosphonic acid (I) [5] and 2-methylaminoethan-1-ol (II). The treatment of compound (III) with 85% aqueous trifluoroacetic acid produces the expected peptidomimetic - diethyl ether {benzoylamino [(2-hydroxyethyl) methylcarbamoyl] methyl} -phosphonic acid (IV) in 57% yield. The behavior of oxazole (III) in the presence of hydrogen chloride under anhydrous conditions was of interest. Not only the 1,3-oxazole cycle is disclosed, but also the hydroxyl group is replaced by a chlorine atom, resulting in the {benzoylamino [(2-chloroethyl) methylcarbamoyl] methyl} -phosphonic acid (V) diethyl ether.
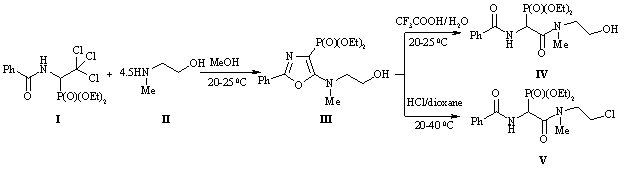
Preliminary biological testing of compounds (III-V) has shown that they exhibit stimulating contractile activity in conditions of coronary heart disease or myocardial infarction.
4-FUNCTIONALIZED 1,3-OXAZOLES CONTAINING A N-MET-HYL-D-GLUCAMINE FRAGMENT AT POSITION 5 /
4-ФУНКЦИОНАЛИЗИРОВАННЫЕ 1,3-ОКСАЗОЛЫ, СОДЕРЖАЩИЕ В ПОЛОЖЕНИИ 5 ФРАГМЕНТ N-МЕТИЛ-D-ГЛЮКАМИНА
ER Abdurakhmanova, EI Lukashuk, AV Golovchenko, VS Brovarets
Keywords: N-methyl-D-glucamine, 1-acylamino-2,2-dihloroacrylic acids, 1-acylamino-2,2,2-trihloroethylphosphonic acids diethyl esters, heterocyclization, 4-functionalized 1,3-oxazoles.
Ключевые слова: N-метил-D-глюкамин, 1-ациламино-2,2-дихлоракриловые кислоты, диэтиловые эфиры 1-ациламино-2,2,2-трихлорэтилфосфоновых кислот, гетероциклизация, 4-функционализированные 1,3-оксазолы.
The interaction of the available derivatives of 1-acylamino-2,2-dichloroacrylic acids, as well as the diethyl esters of 1-acylamino-2,2,2-trichloroethylphosphonic acids with N-methyl-D-glucamine, which leads to previously unknown derivatives of 1,3- oxazole, functionalized at position 4 by nitrile, ester or phosphonyl groups, and containing at position 5 the residue of N-methyl-D-glucamine.
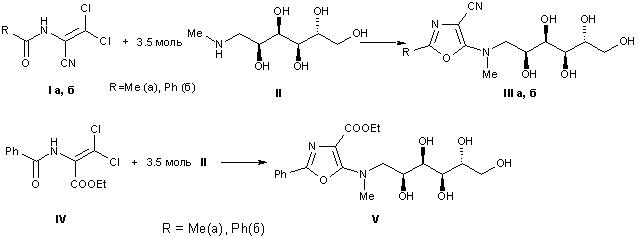
Synthesis and properties of 4-phosphorylated products of 5-hydroxyalkylamino-1,3-oxazole
ER Abdurakhmanova, EI Lukashuk, AV Golovchenko, VS Brovarets
Keywords: amino alcohols, diethyl ethers of 1-acylamino-2,2,2-trichloroethylphosphonic acids, heterocyclization, 1,3-oxazoles, phosphorylated peptidomimetics.
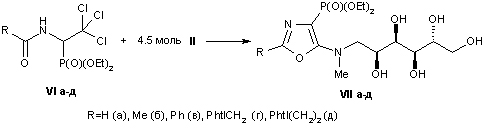
The interaction of diethyl ether derivatives was investigated
1-acylamino-2,2,2-trichloroethylphosphonic acids with various pharmacophore aminoalkanols, which results in previously unknown derivatives of 5-hydroxyalkylamino-1,3-oxazole.
Such compounds have been found to be promising substrates for the production of novel 4-phosphorylated peptidomimetics containing alcohol residues.
Scheme 1

Схема 2

Схема 3

SYNTHESIS OF NEW PHOSPHONOPEPTIDOMIMETICS AND THEIR EFFECT ON CARDIAC OUTPUT
СИНТЕЗ НОВЫХ ФОСФОНОПЕПТИДОМИМЕТИКОВ И ИХ ВЛИЯНИЕ НА ФУНКЦИОНАЛЬНОЕ СОСТОЯНИЕ СЕРДЦА
Э.Р. Абдурахманова, Н.А.Дорофеева, О.И. Лукашук, А.В. Головченко, В.С. Броварец
Key words: heart, contractility, 4-phosphorylated 5-amino-1,3-oxazoles, phosphono-peptido-mimetics, aminoalcohols.
Ключевые слова: сердце, сократимость, 4-фосфорилированные
5-амино-1,3-оксазолы, фосфонопептидомиметики, аминоспирты
The paper presents the synthesis of new phosphorylated peptidomimetics and assess the biological activity of the synthesized compounds on the experimental animals – male rats. On the basis of 1-benzoylamino-2,2,2-trihloroethylphosphonic acids diethyl ester a novel derivative of 1,3-oxazol-4-phosphonic acid diethyl ester containing at position 5 of the oxazole ring an methylaminoethan-1-ol residue was synthesized. The optimal conditions for cleavage of the 1,3-oxazole ring in acidic medium were found to form phosphorylated peptidomimetics. Thus, by treating it with 85% aqueous trifluoroacetic acid {benzoylamino[(2-hydroxyethyl)carbamoyl]methyl} phosphonic acid diethyl ester was obtained, and the action of hydrogen chloride under anhydrous conditions gives {benzoylamino[(2-chloro-ethyl)carbamoyl]methyl} phosphonic acid diethyl ester. The method developed is very convenient and preparative because reactions proceed in mild conditions without formation of undesirable by-products. Peptidomimetics are isolated with high yields and their separation does not require chromatography. Register different functional parameters of cardiac hemodynamics was performed in rats in vivo using mikrokatetora and Millar Pressure-Volume System. Investigation of action of the compounds obtained on the functional state of the heart showed that their introduction intraperitoneally results in a decrease of heart rate and stimulate the contractile activity of the myocardium.
Publications:
1. Kondratyuk K., Lukashuk O., Golovchenko A., Komarov I., Brovarets V., Kukhar V. // Tetrahedron. 2013. Vol. 69. P.6251. DOI:10.1016/j.tet.2013.05.017.
2. Lukashuk O., Kondratyuk K., Golovchenko A., Brovarets V., Kukhar V. // Heteroatom Chem. 2013. Vol.24. P.289. DOI: 10.1002/hc.21093.
3. Lukashuk O.I., Abdurakhmanova E.R., Kondratyuk K.M., Golovchenko O.V., Khokhlov K.V., Brovarets V.S., Kukhar V.P. // RSC Advances. 2015. Vol.5. P.11198. DOI: 10.1039/C4RA13819H.
4. Абдурахманова Э.Р., Лукашук Е.И., Головченко А.В., Пильо С.Г., Броварец В.С. // ЖОХ. 2015. Т. 85. Вып. 4. С. 607; Abdurakhmanova E.R., Lukashuk E.I., Golovchenko A.V., Pil’o S.G., Brovarets V.S. // Zh. Obshch. Khim. 2015. Vol. 85. N 4. P. 851. DOI: 10.1134/S1070363215040143.
Нагороди і сертифікати:

Diploma for the best report in the “Chemistry of organic compounds” in the seventh All-Ukrainian scientific conference with international participation “Chemical problems of the present”


Diploma for the second place in the 7-Ukrainian scientific conference “Chemical Karazinsky Reading 2015”




