Department of chemistry of natural compounds
Завідувач відділу – доктор хімічних наук Смолій Олег Борисович
Google Scholar profile link
https://scholar.google.com.ua/citations?user=_dXe5PMAAAAJ&hl=uk
До складу відділу також входить
Лабораторія № 4.1 Biomedical research,
Керівник відділу – д.б.н., проф. Л.О.Метелиця
The main scientific area of the department is the search for new preparative approaches to the synthesis of functionally substituted polycyclic compounds based on structural analogues of natural substances (caffeine, adenine, guanine) in order to establish a “structure - biological activity” relationship.

Division Employees (2017)
AP Gayevsky, EV Verves, OV Kucher, LV Muzychka,
A. Smolii, G. M. Zinchenko, I. O. Yaremchuk, I. I. Biletsky.
Defenses of dissertations in the Department
• Verves EV Dissertation for the Candidate Degree in Chemical Sciences "Synthesis and Properties of Pyrrolo [2,3-d] Pyrimidine-6-Carboxylic Acid Derivatives" - 2014
• Kucher OV Dissertation for the degree of Candidate of Chemical Sciences “Enantioselective enzymatic separation of 1-cycloalkyl (aryl, hetaryl) ethanol” - 2016.

Employees of the Division (2017)
I.I. Biletsky, O.V. Kucher, L.V. Muzychka, A.B. Smolii, E.V. Verves, G.M. Zinchenko.
Publications in recent years:
• Iryna O. Yaremchuk, Lyubov V. Muzychka, Oleg B. Smolii, Olexandr V. Kucher, Svitlana V. Shishkina Synthesis of novel 1,2-dihydropyrrolo [1,2-a] pyrazin-1(2H)-one derivatives / / Tetrahedron Lett. 2018, Vol. 59, No. 5, P.442-444
• Bohdan A. Chalyk, Andrei A. Isakov, Maryna V. Butko, Kateryna V. Hrebeniuk, Olena V. Savych, Olexandr V. Kucher, Konstantin S. Gavrilenko, Tetiana V. Druzherko, Vladimir S. Yarmolchuk, Sergey Zozulya, Pavel K. Mykhailiuk. Synthesis of 6-Azaspiro [4.3] alkanes Innovative Scaffolds for Drug Discovery // Eur. J. Org. Chem., 2017, №31, P.4530-4542
• Anna N. Zinchenko, Lyubov V. Muzychka, Igor I. Biletskii, Oleg B. Smolii. Synthesis of new 4-amino-substituted 7-iminopyrido [2,3-d] pyrimidines // Chem. Heterocycl. Comp., 2017, Vol.53, №5, P. 589-596
• Zinchenko AN, Muzychka LV, Smolii OB, Bdzhola VG, Protopopov MV, Yarmoluk SM Synthesis and biological evaluation of novel amino-substituted pyrido derivatives [2,3-d] pyrimidine as inhibitors of CK2 protein kinase // Biopolymers and Cell, 2017, Vol. 33, No. 5, P. 367-378
• Lyubov V. Muzychka, Iryna O. Yaremchuk, Oksana V. Muzychka, Oleg B. Smolii 7-Substituted pyrrolo [2,3-d] pyrimidines for the synthesis of new 1-deazapyrimido [1,2,3-cd] purines // French-Ukrainian J. Chem. 2017, Vol.5, №2, P.15-23
• Muzychka LV, Yaremchuk IO, Smolii O. B., Zubatyuk RI, Shishkin OV Synthesis of new pyrroles [2,3-d] Pyrimidine-containing? -Hydroxyphosphonic acids // Journal. org. and the farm. Chemistry, 2016, No. 3, pp. 58-62.
• Zinchenko GM, Muzychka LV, Verves EV, Smolij OB Convenient approach to the synthesis of new 4-substituted pyrido derivatives [2,3-d] pyrimidin-7-one // Journal. org. and the farm. Chemistry, 2016, №4, p. 40-47
• Olexandr V. Kucher, Anastasiya O. Kolodyazhnaya, Oleg B. Smolii, Nadiya K. Nazarenko, Vladimir S. Kubyshkin, Pavel K. Mykhailiuk, Andrey A. Tolmachev. Lipase kinetic enantiomeric resolution of 1-heteroarylethanols // Tetrahedron: Asymmetry, 2016, Vol. 27, No. 7-8, P.341-345
• Muzychka LV, Smolii OB, Biletsky II, Vasilenko OM A convenient approach to the synthesis of substituted 2- (methylamino) quinoline -3-carboxamides // Journal. org. and the farm. Chemistry, 2015, No. 13, pp. 70-78.
• Kucher OV, Kolodyazhnaya AO, Smolij O. B. Enzymatic separation of 2-cycloalkylethanols // Journal. org. and the farm. Chemistry, 2014, No. 12, pp. 65-70.
• Yaremchuk I. O., Kolodyazhnyi O. I, Muzychka L. V., Smolii O. B. Novel approach to the synthesis of Hydroxyphosphonic acid // J. General Chem. 2014, T. 88, No. 1, P.166-168.
• Olexandr V. Kucher, Anastasiya O. Kolodyazhnaya, Oleg B. Smolii, Dmytro V. Prisuazhnyk, Katerina A. Tolmacheva, Olga A. Zaporozhets, Yurii S. Moroz, Pavel K. Mykhailiuk, Andrey A. Tolmachev. Enzyme-catalyzed kinetic resolution of 2,2,2-trifluoro-1- (heteroaryl) ethanols: experimental and docking studies // Eur. J. Org. Chem., 2014, No. 34. P.7692-7698.
• Olexandr V. Kucher, Anastasiya O. Kolodyazhnaya, Oleg B. Smolii, Alexandr I. Boiko, Vladimir S. Kubyshkin, Pavel K. Mykhailyuk, Andrey A. Tolmachev. Enzymatic resolution of chroman-4-ol and its core analogues with Burkholderiacepacia lipase // Tetrahedron Asymetry, 2014, Vol.25, P.63-567.
• Svitlana V. Shishkina, Olexandr V. Kucher, Anastasiya O. Kolodiazhnaya , Oleg B. Smolii, Andrey A. Tolmachev. Crystal structure of (S) -1- (1,3-benzothiazol-2-yl) -2,2,2-trifluoroethanol // Acta Cryst., 2014; E70, o946.
• E. V. Verves, A. V. Kucher, L. V. Muzychka, O. B. Smolii Synthesis of 7-Alkyl-4-amino-7H-pyrrolo [2,3-d] pyrimidine-6-carboxylicAcids // Chem.Heterocycl. Comp., 2013, Vol.48, №12, P. 1844-1852.
• Muzychka LV, Verves EV, Smolii O. B. Bromination of 7-allyl-1H-pyrrolo [2, 3-d] pyrimidine-6-carboxylic acid and its methyl ester // Journal. org. and the farm. Chemistry, 2013, No. 2, pp. 76-79.
Examples of the department's scientific developments
Synthesis of pyrrolo [2,3-d] pyrimidine derivatives with 3-amino-2-hydroxypropyl substituents (Verves EV, Muzychka LV)
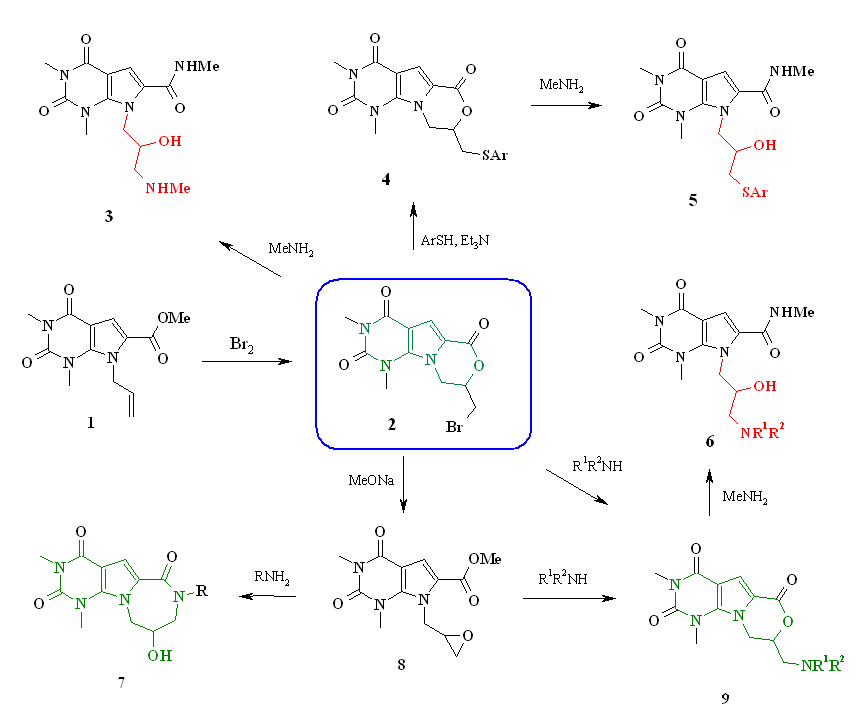
Pyrrolo[2,3-d] pyrimidine derivatives with a carboxyl group at position 6 of the heterocycle proved to be valuable reagents for introducing 2,3-epoxypropyl and 3-amino-2-hydroxypropyl substituents to the nitrogen atom of the pyrrole ring.
In the treatment of bromomethylpyrimido [5 ′, 4 ′: 4,5] pyrrolo [2,1-c] [1,4] oxazine 2 with sodium methylate we obtained ethyl ester of 7-oxyranylmethyl-1H-pyrrolo [2,3-d] pyrimidine-6-carboxylic acids 8. Based on the glycidyl derivative of pyrrolo [2,3-d] pyrimidine 8, a number of representatives of new heterocyclic systems were synthesized - pyrimido [5 ′, 4 ′: 4,5] pyrrolo [1,2-a] [1,4] diazepine 7 and pyrimido [5 ′, 4 ′: 4,5] pyrrolo [2,1-c] [1,4] oxazine 9.
Investigation of the interaction of substrates 2, 8 with aliphatic amines has shown the possibility of using these compounds to obtain pyrrolo [2,3-d] pyrimidine derivatives containing 3-amino-2-hydroxypropyl substituents at position 7 of the heterocycle.
The docking results of some of the compounds obtained indicate the prospects of finding among them effective antagonists of adenosine receptors.
Synthesis of new 7-deazapurine derivatives with alkylphosphoryl substituents (Yaremchuk IO, Muzychka LV)
The development of methods for the synthesis of heterocyclic compounds with phosphorus-containing substituents is associated with the prospect of finding antiviral drugs among them. Particular attention is drawn to beta-hydroxyphosphonates, structural analogues of adefovir (PMEA).
Нами запропонований новий підхід до синтезу фосфорильованих похідних піроло[2,3-d]піримідину.
Вихідною сполукою був вибраний 8-бромометилпіримідо[5′,4′:4,5]піроло[2,1-с][1,4]оксазин,
котрий виявився перспективним реагентом для отримання бета-гідроксифосфонових
кислот. За допомогою нескладних перетворень отримані похідні піроло[2,3-d]піримідину
з 1-гідроксифосфоноетильним замісником в 7 положенні гетероциклічної системи.
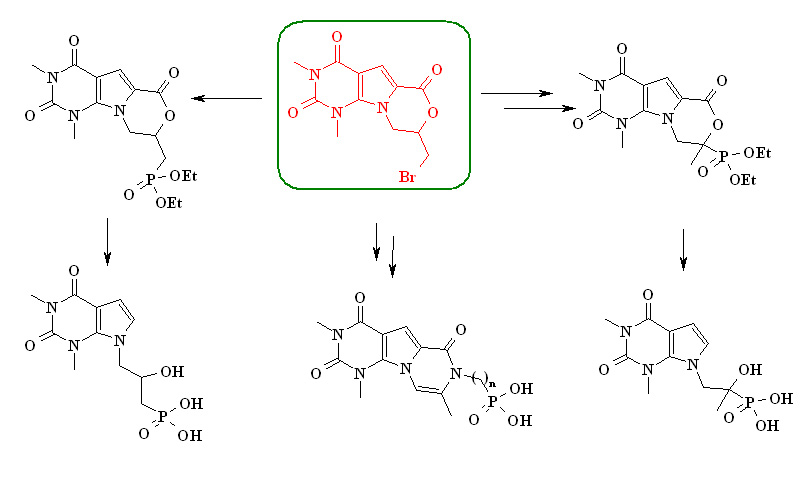
Модифікація піримідо[5′,4′:4,5]піроло[2,1-с][1,4]оксазинового циклу привела до нових похіднихпіразино[1′,2′:1,5]піроло[2,3-d]піримідину з алкілфосфорильними замісниками.
Synthesis of new pyrido [2,3-d]pyrimidin-7-one derivatives (GM Zinchenko, LV Muzychka)
Significant interest in the chemistry of pyrido [2,3-d] pyrimidines is due in recent years to the discovery of selective tyrosine kinase inhibitors, among them, promising targets for the creation of anticancer drugs. Despite the considerable advances in the synthesis of pyrido [2,3-d] pyrimidine derivatives, the search for preparative methods for introducing functional groups into certain positions of the heterocyclic ring remains an urgent task.
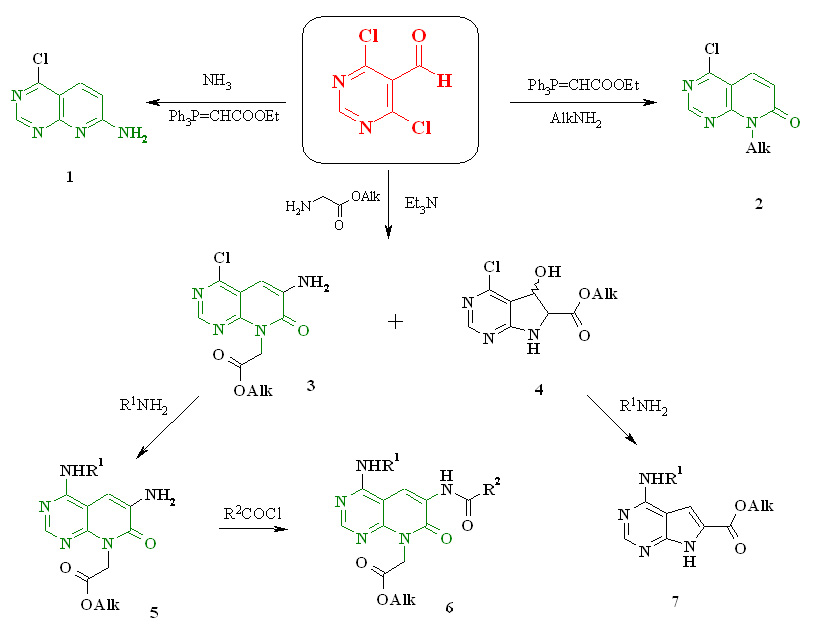
On the basis of 4,6-dichloro-5-formylpyrimidine, a convenient approach to the synthesis of novel pyrido [2,3-d] pyrimidin-7-one 1-6 derivatives containing various nitrogen-containing substituents at positions 4, 6, 7 of the heterocyclic system has been developed. The study of the interaction of 4,6-dichloropyrimidine-5-carbaldehyde with glycine esters led to the development of a one-pot method for obtaining two condensed systems - pyrido [2,3-d] pyrimidine and pyrrolo [2,3-d] pyrimidine. Further modification of the synthesized compounds opens the possibility of obtaining promising pyrido [2,3-d] pyrimidine derivatives for the search for new bioactive substances.
Synthesis and study of the properties of tricyclic compounds with a 1,3-dimethyluracil nucleus (Biletsky II, LV Muzychka)
Of particular interest is the design and synthesis of heterocyclic compounds containing pharmacophores of different heterocyclic nature in their structure. Given the high synthetic potential of pyrimidine derivatives, it remains relevant to design heterocyclic systems that would contain various combinations of pyrimidine nuclei with other heterocyclic systems. Due to the possibility of variation of substituents or additional heterocyclic fragments, they are able to exhibit a wide range of pharmacological activity. Condensed heterocyclic compounds containing the 1,3-dimethyluracil nucleus are promising in this regard.
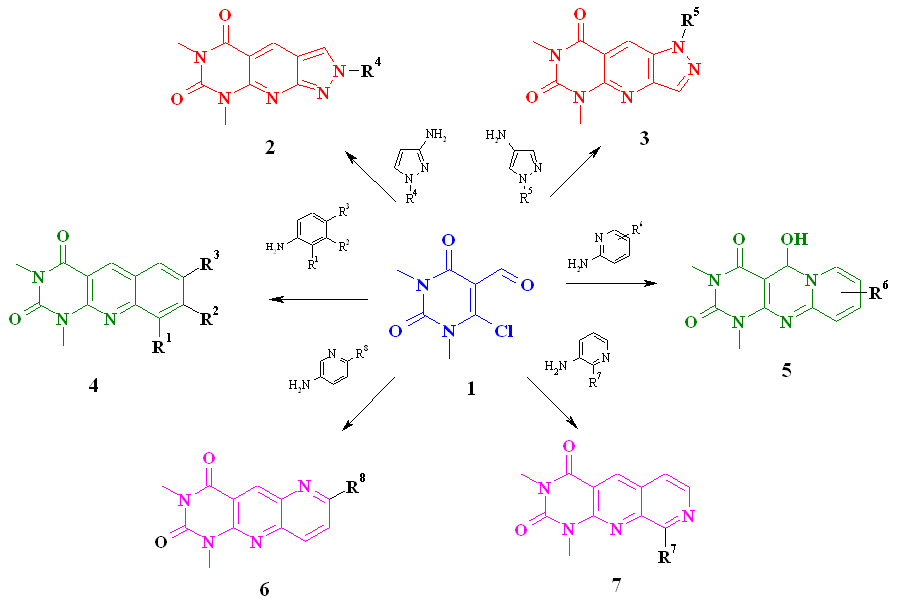
Based on 1,3-dimethyl-5-formyl-6-chlorouracil, a simple approach to the synthesis of tricyclic compounds 2-7 has been developed. New pyrimido [4,5-b] quinoline-2,4(1H,3H) -dione (5-deazaaloxazine), pyrimido [4,5-b] -1,5-naphthyridine-2,4(1H, 3H) -dione and pyrimido [4,5-b]-1,7-naphthyridine-2,4 (1H, 3H) -dione, and pyrazolo [4′,3′: 5,6] pyrido [2,3-d]pyrimidine. The structure of the intermediate products of the cyclization reaction of 6-arylamino-5-formyluracil in 5-deazaaloxazines was investigated. Cyclocondensation of 1,3-dimethyl-5-formyl-6-chlorouracil with substituted 2-, 3-aminopyridines and 3-, 4-, 5-aminopyrazoles was studied.
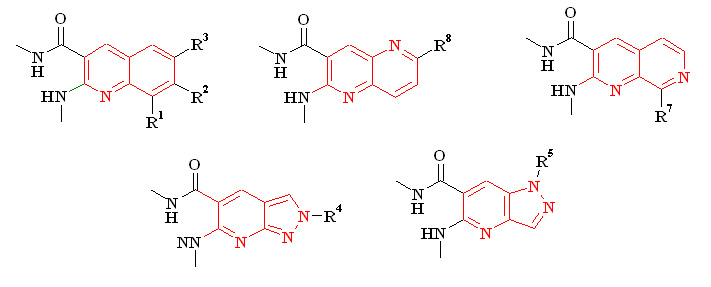
Розроблений простий спосіб синтезу похідних хіноліну, нафтиридину, піразоло[3,4-b]піридину з метиламідними та метиламінозамісниками реакцією гідролітичного розщеплення піримідинового циклу.
Contact Information
02094, Київ, вул Академіка Кухаря, 1
Тел.: +38 (044) 573 27 28
e-mail: Smolii at bpci.kiev.ua

