
Керівник: Михайло Сергійович Фрасинюк, кандидат хімічних наук
Публікації:
1. Development of 6H-Chromeno[3,4-c]pyrido[3′,2′:4,5]thieno[2,3-e]pyridazin-6-ones as Par-4 Secretagogues
M. Frasinyuk, S. Bondarenko, V. Sviripa, R. Burikhanov,
V. Rangnekar, Chunming Liu, and D Watt
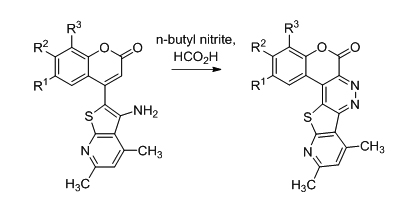
Nitrosation and cyclization of 4-(3-aminothieno[2,3-b]pyridine-2-yl)-2H-chromen-2-ones 1 afforded substituted 6H-Chromeno[3,4-c]pyrido[3′,2′:4,5]thieno[2,3-e]pyridazin-6-ones 2 that inhibited the intermediary filament protein, vimentin, at low micromolar concentrations. This inhibition promoted the secretion of Prostate Apoptosis Response-4 protein (Par-4), which selectively triggered apoptosis in prostate cancer cells such as CWR22Rv1, LNCaP-derivative C4-2B, PC-3 and its aggressive analog, PC-3 MM2.
2. AMINOMETHYLATION OF RELATED NATURAL AURONES
A. Popova, S. Bondarenko, G. Mrug, M. Frasinyuk
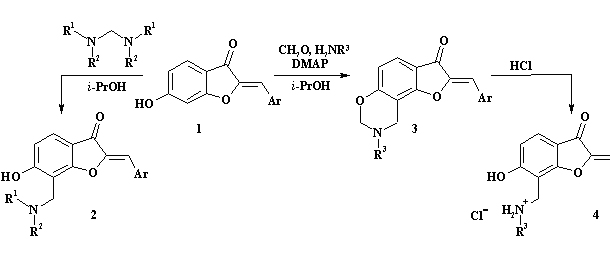
The aim of our work was development of new pathways
for the synthesis of related natural aurones aminomethyl derivatives,
which are containing the residues of secondary and tertiary amines.
The well-known method of synthesis of the Mannich bases using the secondary
amine and formaldehyde in the presence of catalyst was tested. However,
it gave poor chemical yield of aurone Mannich bases which are containing
the residues of tertiatry aliphatic, aromatic and heterocyclic amines
(написано выше) [1]. The good yield for compounds 2 were obtained by heating
of 6-hydroxyaurones 1 with aminals in isopropanol. This procedure allows
us to carry out regiospecific aminomethylation of aurones with formation
of 7-aminomethyl derivatives 2 exclusively.
3. Synthesis of 8-hydroxymethyl and 8-alkoxymethyl 7-hydroxyisoflavone derivatives
G. Mrug, M. Frasinyuk, V. Khilya

Interaction of natural isoflavone Mannich bases with acetic anhydride were synthesized 7-acetoxy-8-acetoxymethyl isoflavones. Alcoholysis of the diacetoxy isoflavones lead to the formation of 8-alkoxymethyl-7-hydroxy isoflavones instead expected 7-hydroxy-8-hydroxymethyl isoflavones. The last compounds were synthesized under hydrolysis of diacetates in dioxane-water medium at the presence of mineral acid.
4. Aminomethylation Of Isoflavones With Cyclic Amino Alcohols
G. P. Mrug, M. S. Frasinyuk, S. P. Bondarenko, V. S. Brovarets, V.P. Khilya
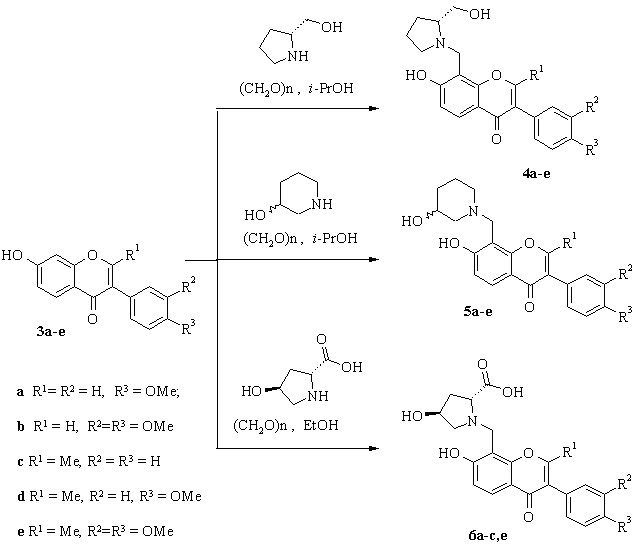
The analogues of chromone alkaloids were synthesized by aminomethylation of natural isoflavones such as formononetin, 2 methylformononetin, cladrin, 7-hydroxy-2-methyl-3-phenylchromone and 2-methyl cladrin derivative with (S)-prolinol, trans-4-hydroxy-L-proline and 3-hydroxypiperidine.
5. Synthesis and Tautomerization of Hydroxylated Isoflavones Bearing Heterocyclic Hemi-Aminals
M. Frasinyuk, S. Bondarenko, V. Khilya, Chunming Liu, D. Watta, and V. Sviripa
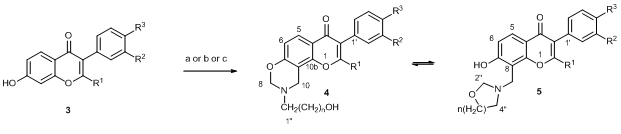
The aminomethylation of hydroxylated isoflavones with 2-aminoethanol, 3-amino-1-propanol, 4-amino-1-butanol, and 5-amino-1-pentanol in the presence of excess formaldehyde led principally to 9-(2-hydroalkyl)-9,10-dihydro-4H,8H-chromeno[8,7-e][1,3]-oxazin-4-ones 4 and/or the tautomeric 7-hydroxy-8-(1,3-oxazepan-3-ylmethyl)-4H-chromen-4-ones 5. The ratio of these tautomers was dependent on solvent polarity, electronic effects of aryl substituents in the isoflavone and the structure of the amino alcohol. NMR studies confirmed the interconversion of tautomeric forms.
6. Feature of aminomethylation of esculetin derivatives
M. Frasinyuk
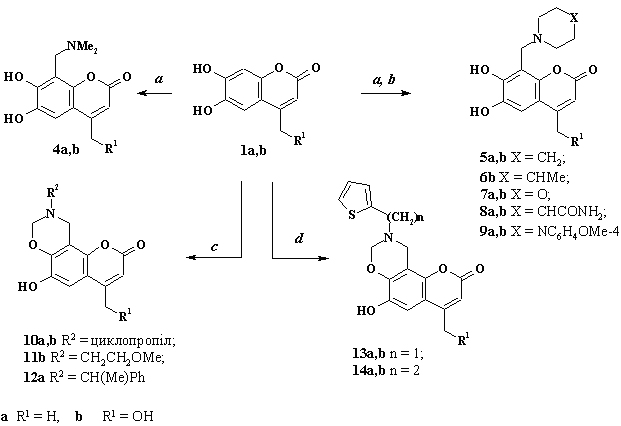
4-Subsituted esculetine derivatives were synthesized
by condensation of pyrogalol A ethyl acetoacetate or ethyl 4-chloroacetoacetate
under Pechmann’s reaction. 4-Hydroxymethylesculetin was synthesized by
acetylation of 4-chloromethylesculetin with acetic anhydride in presence
of potassium acetate and subsequent deacylation in ethanol. Aminomethylation
of 4-methyl- and 4-hydroxymethyl esculetin derivatives was studied at
various reaction conditions. A different 8-aminomethyl esculetine derivatives
and 2H,8H-chromeno[8,7-e][1,3]oxazin-2-ones were synthesized with good
or excellent yield.
7. ALKALOID-FLAVONOID CONJUGATES
S. Bondarenko, V. Khilya, A. Popova, M. Frasinyuk
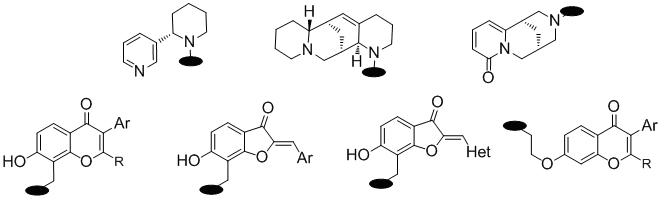
The interaction of 7-isoflavonoids with alkaloids in
Mannich reaction was studied for the synthesis of semi-synthetic related
alkaloid-flavonoid conjugates. The possibility of applying of cytisine,
anabasine, and aloperine as amine was shown for aminomethylation of 7-hydroxyisoflavones,
6-hydroxyaurones, and related 6-hydroxy-3-hetarylbenzofuran-3-ones with
paraformaldehyde. In all cases using of 4-(dimetylamino)pyridine was required.
The aminomethylation of 7-hydroxyisoflavones with alkaloids was regioselective
and led to formation of 8-alkaloid-methyl-7-hydroxyisoflavones. The similar
reaction with 6-hydroxyaurones and their hetero analogues led to 7-aminomethyl
substituted benzylideno 6-benzofuran-3-ones.
Another way for the synthesis of alkaloid-isoflavone conjugates was alkylation
of cytisine or aloperine with 7-(2-bromoethoxy)isoflavones, which were
obtained by alkylation with 1,2-dibromoethane. In this cases C2-linked
hybrids were obtained.
Thus, we synthesized various derivatives containing flavonoid and cytisine,
aloperin, or anabasine moieties connected by one or two methylene groups
in the position 8 or in O-7 of the chromone ring respectively. Another
type of alkaloid-flavonoid conjugates are presented as 7-aminomethyl-6-hydroxyaurones.
8. REGIOSPECIFIC SYNTHESIS OF 4,5-DIARYLISOXAZOLES BEARING CYTISINE MOIETY
S. Bondarenko, M. Frasinyuk, V. Khily, V. Vinogradova
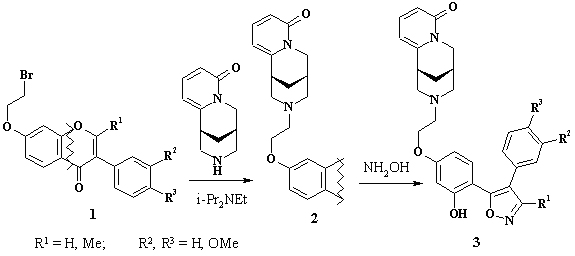
Thus, reaction of hydroxylamine hydrochroride
with compounds 2 (R1 = Me) in pyridine led to formation only 2-methyl-3-aryl-4-(4-(2-(cytisin-12yl)ethoxy-2-hydroxyphenyl))isoxazoles
3.
In case of 2-terminated isoflavones 2 the similar reaction led to formation
of both regio-isomeric isoxazoles. It was found, the formation of target
regio-isomeric isoxazoles 3 (R1 = H) is possible in ethanol in presense
of 4-methylmorpholine, which provide nucleophile attack in C-2 chromone
ring.
The structures of 4,5-diarylisoxazoles 3 were confirmed by HSQC and HMBC
spectra. In case of 2-methyl isoxazole derivatives with HMBC spectra were
identified that 2-Me group is linked with carbon at 158-160 ppm, and carbon
at 163-165 ppm is linked with phenolic substituent. The similar results
were observed for compounds 3 (R1 = H). It was identified with HSQC spectra,
CH carbon shift was 151-152 ppm. These data are confirming regiospecific
formation 4,5-diarylisoxazoles 3.
9. APPLYING OF 4-CHLOROMETHYLCOUMARINS AS SYNTHONES FOR THE SYNTHESIS OF FUSED HETEROCYCLIC SYSTEMS
S.P. Bondarenko, M.S. Frasinyuk
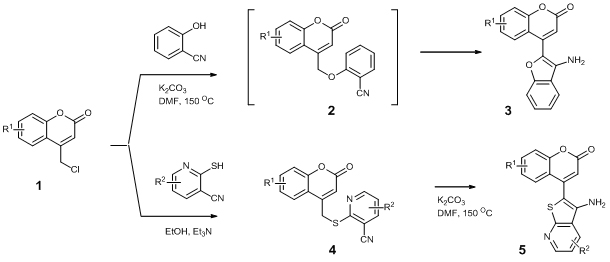
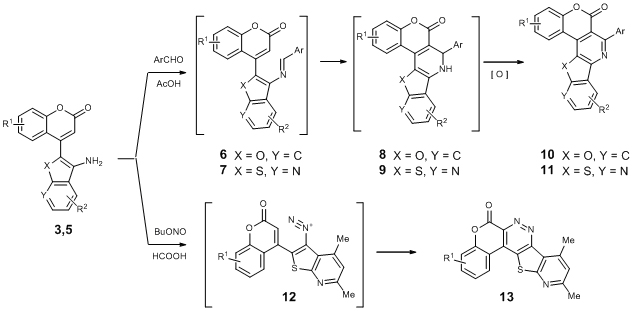
We developed new methods for the synthesis of
various fused coumarins using 4-chloromethylcoumarins as versatile starting
compounds. Alkylation of 2-cyanophenol with 4-chloromethylcoumarins led
to one-pot formation of 4-(3-aminobenzofuran-2-yl)coumarins 3 via formation
of 4-aryloxymethylcoumarins 2 and intramolecular cyclization cyano and
active methylene groups. The similar reaction involving 2-mercapto-3-cyanopyridines
required alkylation in EtOH with presence of organic base and isolation
of S-alkylpyridines 4. 4-(3-Aminothieno[2,3-b]pyridin-2-yl)-2H-chromen-2-ones
(5) were synthesized by subsequent cyclization compounds 4 in DMF with
presence of K2CO3 as base (scheme 1).
Unusual condensation of compounds 3,5 with aldehydes
led to formation of fused pyridino[3,4-c]coumarins 10, 11 which is results
of formation of the Shiff’s bases 6,7, intramolecular [5+1] cycloaddition
and oxidation of dihydropyridino[3,4-c]coumarins 8, 9 [2,3].
Interaction of compounds 5 with BuONO in formic acid led to synthesis
of diazo compounds 12 with further intramolecular ring-closure reaction
and formation of 6H-chromeno[3,4-c]pyrido[3′,2′:4,5]thieno[2,3-e]pyridazin-6-ones
13 [4] (scheme 2).
Список останніх публікацій
1. M. S. Frasinyuk, G. P. Mrug, S. P. Bondarenko, V. M. Sviripa, W. Zhang, X. Cai, M. V. Fiandalo, J. L. Mohler, C. Liu, D. S. Watt, Application of Mannich Bases to the Synthesis of Hydroxymethylated Isoflavonoids As Potential Antineoplastic Agents, Org. Biomol. Chem., 2015, 13 (46), 12292-11301. http://dx.doi.org/10.1039/C5OB01828E
2. М. С. Фрасинюк, Особливості амінометилювання похідних ескулетину, Ukr. Вioorg. Acta, 2015, 13 (1), 3 – 8.
3. M. S. Frasinyuk, S. P. Bondarenko, V. M. Sviripa, R. Burikhanov, V. M. Rangnekar, C. Liu, D. S. Watt, Development of 6H-Chromeno[3,4-c]pyrido[3′,2′:4,5]thieno[2,3-e]pyridazin-6-ones as Par-4 Secretagogues, Tetrahedron Lett., 2015, 56, 23, 3382 – 3384. http://dx.doi.org/10.1016/j.tetlet.2015.01.028
4. S. P. Bondarenko, М. S. Frasinyuk, V. P. Khilya, Synthesis of aloperine-containing Mannich bases of isoflavones, Chem. Nat. Compd., 2014, 51 (4), 643 – 645. http://dx.doi.org/10.1007/s10600-015-1375-8
5. T. V. Shokol, N. V. Gorbulenko, M. S. Frasinyuk, V. P. Khilya, Synthesis of 6-(3-Pyrazolyl)-4-Methylumbelliferone Derivatives Substituted on the Pyrazole Ring, Chem. Nat. Compd., 2014, 51 (4), 630 – 633. http://dx.doi.org/10.1007/s10600-015-1371-z
6. M. S. Frasinyuk, S. P. Bondarenko, V. P. Khilya, C. Liu, D. S. Watt, V. M. Sviripa, Synthesis and Tautomerization of Hydroxylated Isoflavones Bearing Heterocyclic Hemi-Aminals, Org. Biomol. Chem., 2015, 13 (4), 1053-1067. http://dx.doi.org/10.1039/c4ob02137a
7. І. С. Безверха, Т. М. Пантелеймонова, М. У. Заїка, Л. Б. Шарабура, М. С. Фрасинюк, В. П. Хиля, Антидепресивна дія ізофлавону 5/09 при тривожній депресії у самців мишей, Пробл. Старения и долголетия, 2014, № 2, 101-112.
8. Патент №105959, Україна, МПК (2014.01) С07D311/36, A61/K 31/00, C07D 295/04. 7-[2-(4-Етилпіперазин-1-іл)етокси]-2-метил-3-(4-хлорофеніл)-4Н-хромен-4-он та його застосування / І. С. Безверха, С. П. Бондаренко, М. У. Заїка, Т. М. Пантелеймонова, М. С. Фрасинюк, В. П. Хиля, Л. Б. Шарабура; патентовласник ДУ “Інститут геронтології ім. Д. Ф. Чеботарьова НАМНУ” – заявка № а20123435; заявл. 26.11.2012; опублік. 26.05.2014, Бюл. № 10.
9. G. P. Mrug, M. S. Frasinyuk, S. P. Bondarenko, V. S. Brovarets, V. P. Khilya, Aminomethylation of isoflavones with cyclic amino alcohols, Ukr. Вioorg. Acta, 2014, 12 (2), 10 – 14. http://www.bioorganica.org.ua/UBAdenovo/vol_12_2.htm
10. G. P. Mrug, M. S. Frasinyuk, V. P. Khilya, Synthesis of 8-hydroxymethyl and 8-alkoxymethyl 7-hydroxyisoflavone derivatives, Ukr. Вioorg. Acta, 2014, 12 (1), 24 – 28. http://www.bioorganica.org.ua/UBAdenovo/vol_12_1.htm
11. M. S. Frasinyuk, Synthesis and aminomethylation of 3-substituted 6-hydroxy-1,2-benzisoxazoles, Chem. Heterocycl. Compd., 2014, 50 (11), 1616-1623. http://dx.doi.org/10.1007/s10593-014-1631-z
12. S. P. Bondarenko, М. S. Frasinyuk, V. I. Vinogradova, V. P. Khilya, Synthesis of 4-aryl-3-[2-hydroxy-4-(2-cytisin-12-ylethoxy)phenyl]pyrazoles, Chem. Nat. Compd., 2014, 50 (5), 889-891. http://dx.doi.org/10.1007/s10600-014-1107-5
13. M. S. Frasinyuk, S. Kwiatkowski, J. M. Wagner, T. J. Evans, R. W. Reed, K. V. Korotkov, D. S. Watt, Pentapeptide Boronic Acid Inhibitor of Mycobacterium tuberculosis MycP1 Protease, Bioorg. Med. Chem. Lett., 2014, 24 (15), 3546-3548. http://dx.doi.org/10.1016/j.bmcl.2014.05.056
14. А. Hamza, J. M. Wagner, T. J. Evans, M. S. Frasinyuk, S. Kwiatkowski, C.-G. Zhan, D. S. Watt, K. V. Korotkov, Novel Mycosin Protease MycP1 Inhibitors Identified by Virtual Screening and 4D Fingerprints, J. Chem. Inf. Model., 2014, 54 (4), 1166 – 1173. http://dx.doi.org/10.1021/ci500025r
15. M. S. Frasinyuk, S. P. Bondarenko, N. V. Gorbulenko, A. V. Turov, V. P. Khilya, Cyclic carboxylic anhydrides as new reagents for formation of chromone ring, J. Heterocycl. Chem., 2014, 51, 768 – 774. http://dx.doi.org/10.1002/jhet.1721
16. S. P. Bondarenko, E.V. Podobii, М. S. Frasinyuk, V. I. Vinogradova, V. P. Khilya, Synthesis of Cytisine Derivatives of Flavonoids. 4. Synthesis of 3-aryl-7-[2-(cytisin-12-yl)ethoxy]coumarins. Chem. Nat. Compd., 2014, 50, 420 – 423. http//dx.doi.org/10.1007/s10600-014-0975-z
17. M. S. Frasinyuk, G. P. Mrug, S. P. Bondarenko, V. P. Khilya, V. S. Brovarets, Antitumor activity of flavonoid Mannich bases, Ukrainica Bioorganica Acta, 2013, 11, 2, 3-7 (Ukr).http://www.bioorganica.org.ua/UBAdenovo/vol_11_2.htm
18. S. P. Bondarenko, M. S. Frasinyuk, Synhsis of aminomethyl derivatives of chrysin, Chem. Nat. Compd., 2013, 49, 841 – 844. http://dx.doi.org/10.1007/s10600-013-0760-4
19. S. P. Bondarenko, O. N. Miroshnikov, M. S. Frasinyuk, V. P. Khilya, Synthesis of 4-aryl-5-[2-hydroxy-4-?-(N,N-dialkylamino)ethoxyphenyl]isoxazoles, Chem. Nat. Compd., 2013, 49, 826 – 829. http://doi.org/10.1007/s10600-013-0757-z
20. I. S. Bezverha, T. M. Panteleymonova, M. U. Zaika, L. B. Sharabura, М. S. Frasinyuk, V. P. Khilya, S. P. Bondarenko, Antidepressant and neuroleptic action of new derivatives nitrogen-containing isoflavones, Problemy stareniy i dolgoletiya, 2013, 22, 2, 145 – 155 (Ukr).
21. G. P. Mrug, S. P. Bondarenko, V. P. Khilya, M. S. Frasinyuk, Synthesis and aminomethylation of 7-hydroxy-5-methoxyisoflavones, Chem. Nat. Compd., 2013, 49, 235 – 241. http://dx.doi.org/10.1007/s10600-013-0570-8
22. S. P. Bondarenko, М. S. Frasinyuk, V. I. Vinogradova, V. P. Khilya, Synthesis of Cytisine Derivatives of Flavonoids. 3. Synthesis of 7-[2-(cytisin-12-yl)ethoxy]isoflavones. Chem. Nat. Compd., 2012, 48, 970 – 973. http://dx.doi.org/10.1007/s10600-013-0441-3
23. S. P. Bondarenko, М. S. Frasinyuk, V. P. Khilya, T. M. Panteleymonova, I. S. Bezverkha, Synthesis and antioxidant activity of 6- and 7-hydroxy-3-arylcoumarins, Ukrainica Bioorganica Acta, 2012, 10, 42 – 47. http://www.bioorganica.org.ua/UBAdenovo/vol_10_1.htm
24. S. V. Gorelov, S. P. Bondarenko, М. S. Frasinyuk, Synthesis and Properties of 4-(3-Aminothieno[2,3-b]pyridin-2-yl)coumarins, Chem. Heterocycl. Compd., 2012, 48, 955-962. http://dx.doi.org/10.1007/s10593-012-1083-2
25. M. S. Frasinyuk, G. P. Mrug, O. D. Fedoryak, S. P. Bondarenko, Synthesis of aminoacyl derivatives of formononetin and cladrin, Chem. Nat. Compd., 2012, 48, 570-573. http://dx.doi.org/10.1007/s10600-012-0313-2.
26. S. P. Bondarenko, М. S. Frasinyuk, A. I. Galayev, V. I. Vinogradova, New flavonoid-contain derivatives of lupinine. Chem. Nat. Compd., 2012, 48, 234-237. http://dx.doi.org/10.1007/s10600-012-0212-6
27. М. S. Frasinyuk, S. P. Bondarenko, V. P. Khilya, Chemistry of 3-Hetarylcoumarins. 3. Synthesis and Aminomethylation of 7′-Hydroxy-3,4′-bicoumarins. Chem. Heterocycl. Compd., 2012, 48, 422- 426. http://dx.doi.org/10.1007/s10593-012-1009-z
28. S. P. Bondarenko, М. S. Frasinyuk, V. P. Khilya, Synthesis of Aminomethyl Derivatives of Sophoricoside. Chem. Nat. Compd., 2012, 48, 26-29. http://dx.doi.org/10.1007/s10600-012-0151-2
29. S. P. Bondarenko, М. S. Frasinyuk, V. I. Vinogradova, V. P. Khilya, Synthesis of Cytisine Derivatives of Flavonoids. 2. Aminomethylation of 7-hydroxyisoflavones. Chem. Nat. Compd., 2011, 47, 604-607. http://dx.doi.org/10.1007/s10600-011-0006-2
30. М. S. Frasinyuk, S. P. Bondarenko, O.V. Shablykina, V. P. Khilya, Formylation of 5-Hydroxybenzofuran Derivatives and Synthesis of Furo[3,2-f]coumarins based them. Chem. Heterocycl. Compd., 2011, 47, 1155- 1163. http://dx.doi.org/10.1007/s10593-011-0886-x
31. S. P. Bondarenko, М. S. Frasinyuk, V. I. Vinogradova, V. P. Khilya, Synthesis of flavonoid derivatives of cytisine. 1. Aminomethylation of 7-hydroxy-3-arylcoumarins. Chem. Nat. Compd., 2010, 46, 771-773. http://dx.doi.org/10.1007/s10600-010-9737-8
32. S. P. Bondarenko, М. S. Frasinyuk, V. P. Khilya, Aminomethylation of 3-Aryl -7-hydroxycoumarins. Chem. Heterocycl. Compd., 2010, 46, 529-535. http://dx.doi.org/10.1007/s10593-010-0541-y
33. S. P. Bondarenko, М. S. Frasinyuk, V. P. Khilya, Features of Aminomethylation of 7-Hydroxy-4′-Fluoroisoflavones with Primary Amines, Chem. Heterocycl. Compd., 2010, 46, 146-150. http://dx.doi.org/10.1007/s10593-010-0485-2
