
Group of synthesis and study of phosphoruscontaining heterocyclic compounds properties
 |
Head of group Oleksandr Golovchenko, Ph.D. |
Publications :
SYNTHESIS OF NEW PHOSPHONOPEPTIDOMIMETICS /
E.R. Abdurakhmanova, O.I. Lukashuk, A.V. Golovchenko, V.S. Brovarets
Keywords: 4-phosphorylated 5-amino-1,3-oxazoles, phosphonopeptidomimetics, aminoalcohols
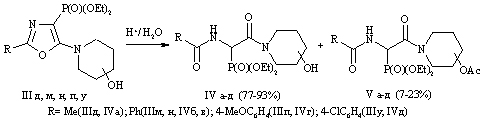
One of the promising methods for the preparation of phosphorylated peptide mimetics is acid decomposition of 5-amino-1,3-oxazole ring. With a view to the introduction of the ethanol fosfonopeptidnuyu chain fragment we obtained diethyl ether {5-[(2-hydroxyethyl)methylamino]-2-phenyl-1,3-oxazol-4-yl}phosphonic acid (III) from diethyl 1-benzoylamino-2,2,2-trihloretilfosfonovoy acid (I) [5] and 2-metilaminoetan-1-ol (II). In the treatment of compound (III) of 85% aqueous trifluoroacetic acid produced the expected peptidomimetic - diethyl ether {benzoylamino[(2-hydroxyethyl)methylcarbamoyl]methyl}-phosphonic acid (IV) with a yield of 57%. Interesting was the behavior of oxazole (III) in the presence of hydrogen chloride under anhydrous conditions. When this occurs not only disclosing 1,3-oxazole ring, but replacing the hydroxy group by chlorine, resulting in diethyl ether of {benzoylamino[(2-chloroethyl)methylcarbamoyl]methyl}-phosphonic acid (V) obtained .
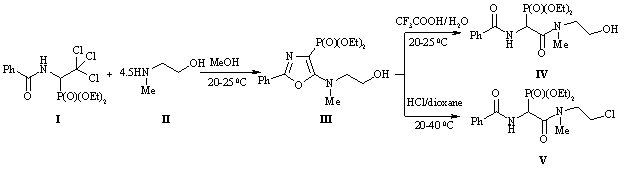
Preliminary biological tests of the compounds (III-V) have shown that they exhibit catalytic activity in terms contractility of ischemic heart disease or myocardial infarct.
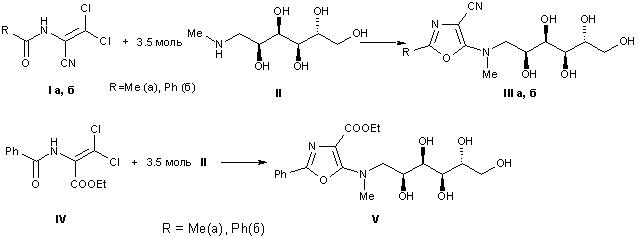
4-FUNCTIONALIZED 1,3-OXAZOLES CONTAINING A N-MET-HYL-D-GLUCAMINE
FRAGMENT AT POSITION 5 /
E.R. Abdurakhmanova, O.I. Lukashuk, A.V. Golovchenko, S.G Pilyo, V.S. Brovarets
Keywords: N-methyl-D-glucamine, 1-acylamino-2,2-dihloroacrylic acids, 1-acylamino-2,2,2-trihloroethylphosphonic acids diethyl esters, heterocyclization, 4-functionalized 1,3-oxazoles
Interactions of available derivatives of 1-acylamino-2,2-dihlorakrylic acids and the diethyl ester of 1-acylamino-2,2,2-trihloretilfosphonic acids with N-methyl-D-glucamine, which leads to a previously unknown 1,3-oxazole derivative functionalized at the 4-position nitrile, ester or phosphonyl group, and containing residue in position 5 N-methyl-D-glucamine investigated.
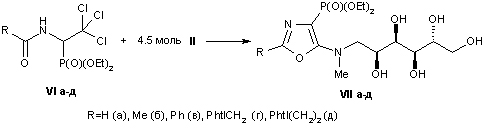
Synthesis and properties of 4-phosphorylated derivatives of 5-hydroxyethylamino-1,3-oxazole
E.R. Abdurakhmanova, O.I. Lukashuk, A.V. Golovchenko, V.S. Brovarets
Keywords: aminoalcohols, esters of 1-diethyl-2,2,2-acylamino acids trihloretilfosfonovyh, heterocyclization, 1,3-oxazoles, phosphorylated peptidomimetics.
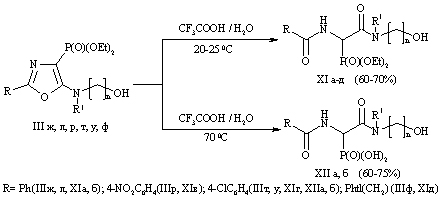
The interaction of the diethyl ester derivatives of 1-acylamino-2,2,2-trihloretilfosfonovyh acids with different pharmacophoric aminoalkanol which leads to a previously unknown derivatives of 5-hydroxyalkylamino-1,3-oxazole. We found that these compounds are promising for new substrates 4-phosphorylated peptidomimetics containing alcohol residues.
Scheme 1
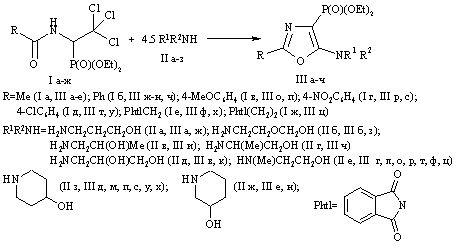
Scheme 2
Scheme 3
SYNTHESIS OF NEW PHOSPHONOPEPTIDOMIMETICS AND THEIR
EFFECT ON CARDIAC OUTPUT
E.R. Abdurakhmanova, N.A.Dorofeeva, O.I. Lukashuk, A.V. Golovchenko, V.S. Brovarets
Key words: heart, contractility, 4-phosphorylated 5-amino-1,3-oxazoles, phosphono-peptido-mimetics, aminoalcohols.
The paper presents the synthesis of new phosphorylated peptidomimetics and assess the biological activity of the synthesized compounds on the experimental animals - male rats. On the basis of 1-benzoylamino-2,2,2-trihloroethylphosphonic acids diethyl ester a novel derivative of 1,3-oxazol-4-phosphonic acid diethyl ester containing at position 5 of the oxazole ring an methylaminoethan-1-ol residue was synthesized. The optimal conditions for cleavage of the 1,3-oxazole ring in acidic medium were found to form phosphorylated peptidomimetics. Thus, by treating it with 85% aqueous trifluoroacetic acid {benzoylamino[(2-hydroxyethyl)carbamoyl]methyl} phosphonic acid diethyl ester was obtained, and the action of hydrogen chloride under anhydrous conditions gives {benzoylamino[(2-chloro-ethyl)carbamoyl]methyl} phosphonic acid diethyl ester. The method developed is very convenient and preparative because reactions proceed in mild conditions without formation of undesirable by-products. Peptidomimetics are isolated with high yields and their separation does not require chromatography. Register different functional parameters of cardiac hemodynamics was performed in rats in vivo using mikrokatetora and Millar Pressure-Volume System. Investigation of action of the compounds obtained on the functional state of the heart showed that their introduction intraperitoneally results in a decrease of heart rate and stimulate the contractile activity of the myocardium.
Recent Publications:
1. Kondratyuk K., Lukashuk O., Golovchenko A., Komarov I., Brovarets V., Kukhar V. // Tetrahedron. 2013. Vol. 69. P.6251. DOI:10.1016/j.tet.2013.05.017.
2. Lukashuk O., Kondratyuk K., Golovchenko A., Brovarets V., Kukhar V. // Heteroatom Chem. 2013. Vol.24. P.289. DOI: 10.1002/hc.21093.
3. Lukashuk O.I., Abdurakhmanova E.R., Kondratyuk K.M., Golovchenko O.V., Khokhlov K.V., Brovarets V.S., Kukhar V.P. // RSC Advances. 2015. Vol.5. P.11198. DOI: 10.1039/C4RA13819H.
4. Àáäóðàõìàíîâà Ý.Ð., Ëóêàøóê Å.È., Ãîëîâ÷åíêî À.Â., Ïèëüî Ñ.Ã., Áðîâàðåö Â.Ñ. // ÆÎÕ. 2015. Ò. 85. Âûï. 4. Ñ. 607; Abdurakhmanova E.R., Lukashuk E.I., Golovchenko A.V., Pil'o S.G., Brovarets V.S. // Zh. Obshch. Khim. 2015. Vol. 85. N 4. P. 851. DOI: 10.1134/S1070363215040143.
Awards:

Diploma for the best report in the "Chemistry of organic compounds" in the seventh All-Ukrainian scientific conference with international participation "Chemical problems of the present"


Diploma for the second place in the 7-Ukrainian scientific conference "Chemical Karazinsky Reading 2015"




© V.P. Kukhar IBOPC NAS Ukraine 2000-2019