The Department of Mechanisms of Bioorganic Reactions (until 2001 – Department of Photosynthesis Chemistry) was established in 1963 based on the biocatalysis group of the Department of Organic Reaction Mechanisms of the Institute of Organic Chemistry of the National Academy of Sciences of Ukraine. For many years, the Department was headed by a Corresponding Member of the National Academy of Sciences of Ukraine, Doctor of Chemical Sciences, Professor O.O. Yasnikov. Since 1999, the Department has been headed by Doctor of Chemical Sciences, Professor A.I. Vovk (Corresponding Member of the National Academy of Sciences of Ukraine since 2009). The Department conducts fundamental scientific research in the field of bioorganic chemistry aimed at elucidating the mechanisms of bioorganic transformations, synthesising and studying the properties of new bioactive compounds, and establishing the relationship between their structure and activity.
The main areas of scientific activity of the Department include studying the mechanisms of model reactions and bioactivity mechanisms of natural and synthetic organic compounds, designing and synthesising potentially bioactive organic molecules, establishing the relationship between their structure and activity, and predicting bioactivity using computer modelling methods. The objects of research are natural and synthetic heterocyclic and organophosphorus compounds, as well as therapeutically important hydrolases, transferases, oxidoreductases, and other protein structures, which serve as potential targets. Recent work has focused on investigating the properties of bioactive structural analogues of vitamin B1 and searching for, designing, synthesising and studying inhibitors of non-specific alkaline phosphatases, protein tyrosine phosphatases, cholinesterases, glutathione-S-transferases, xanthine oxidase, α-glucosidase and other enzymes. The study of antioxidants and spin probes continues. Along with experimental approaches in vitro, the Department's research utilises methods of molecular docking, molecular dynamics, and QSAR.
Scientists of the Department
Oleksandr Kobzar, Candidate of Chemical Sciences, Senior Researcher
https://www.scopus.com/authid/detail.uri?authorId=57205476268
https://orcid.org/0000-0003-4370-7041
https://scholar.google.com.ua/citations?user=cmcLKhAAAAAJ&hl=uk
https://nauka.gov.ua/researchers/rs.3WmphPcT/
Vsevolod Tanchuk, Candidate of Chemical Sciences, Senior Research Fellow
https://orcid.org/0000-0001-9055-870X
https://www.scopus.com/authid/detail.uri?authorId=6701780283
https://scholar.google.com/citations?user=W5UXRO0AAAAJ&hl=uk&oi=sra
Oksana Muzychka, Candidate of Chemical Sciences, Research Fellow
https://www.scopus.com/authid/detail.uri?authorId=6506102477
https://scholar.google.com/citations?hl=uk&user=cQsR9vMAAAAJ
https://orcid.org/0000-0001-9856-7221
Vladyslav Buldenko, Candidate of Chemical Sciences, Research Fellow
https://www.scopus.com/authid/detail.uri?authorId=57205554823
https://orcid.org/0000-0003-2999-9826,
https://scholar.google.com/citations?user=0ePOahkAAAAJ
Olexandr Sukhoveev, Candidate of Chemical Sciences, Research Fellow
https://www.scopus.com/authid/detail.uri?authorId=57074049200
https://orcid.org/0000-0001-9949-2188
https://scholar.google.com.ua/citations?user=cabJcfsAAAAJ&hl=uk
Yurii Shulha, PhD, Junior Research Fellow
https://www.scopus.com/authid/detail.uri?authorId=57511352600
https://orcid.org/0000-0002-1073-0600
https://scholar.google.com/citations?user=4OXTXUMAAAAJ&hl=uk
Alona Beiko, PhD, Junior Research Fellow
https://www.scopus.com/authid/detail.uri?authorId=58400439000
https://orcid.org/0000-0003-1119-988X
https://scholar.google.com.ua/citations?user=4LN3jWQAAAAJ&hl=uk
The most important publications of the Department researchers
Myshko, A. S., Mrug, G. P., Bondarenko, S. P., Demydchuk, B. A., Kobzar, O. L., Buldenko, V. M., Vovk A.I., Frasinyuk, M. S. 2025. Divergent synthesis of novel 3 (5)‐aminoazole–benzopyrone hybrids and their evaluation as α‐glucosidase Inhibitors. ChemMedChem, 20(5), e202400525. https://doi.org/10.1002/cmdc.202400525

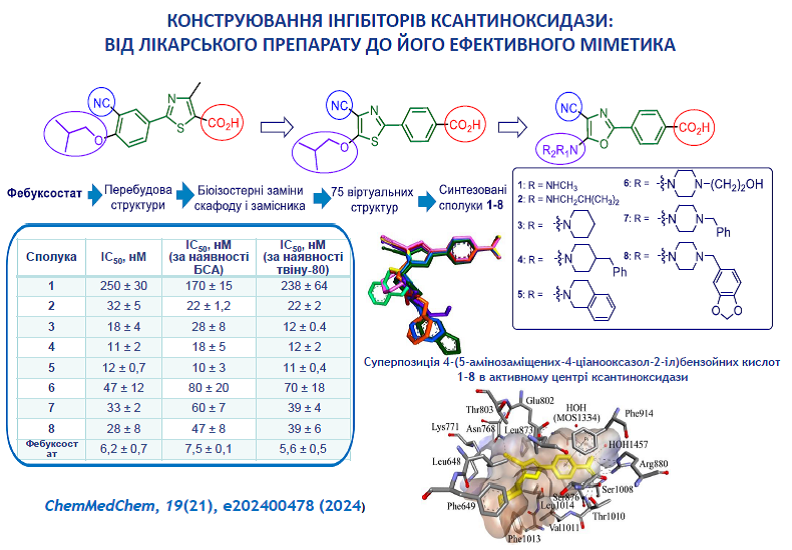
2. Kobzar, O., Beiko, A., Merzhyievskyi, D., Shablykin, O., Brovarets, V., Tanchuk, V., Vovk, A. Design, synthesis, and xanthine oxidase inhibitory activity of 4‐(5‐aminosubstituted‐4‐cyanooxazol‐2‐yl) benzoic acids. ChemMedChem 2024, 19(21), e202400478. https://doi.org/10.1002/cmdc.202400478

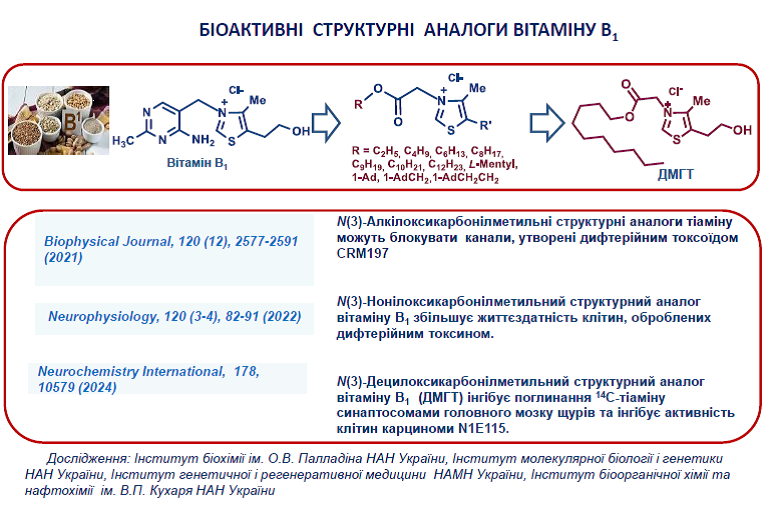
3. Parkhomenko, Y. M., Vovk, A. I., Protasova, Z. S., Pylypchuk, S. Y., Chorny, S. A., Pavlova, O. S., Pylypchuk, Yu., Stepanenko, S. P. Thiazolium salt mimics the non-coenzyme effects of vitamin B1 in rat synaptosomes. Neurochemistry International 2024, 178, 105791. https://doi.org/10.1016/j.neuint.2024.105791
4. Myshko, A. S., Mrug, G. P., Bondarenko, S. P., Kondratyuk, K. M., Kobzar, O. L., Buldenko, V. M., Vovk, A.I., Frasinyuk, M. S. Trapping of thermally generated ortho-and para-quinone methides by imidazoles and pyrazoles: a simple route to green synthesis of benzopyrone-azole hybrids and their evaluation as α-glucosidase inhibitors. RSC advances 2024, 14(38), 27809-27815. https://doi.org/10.1039/D4RA05230G
5. Zhirnov, V., Shablykin, O., Chumachenko, S., Kornii, Y., Keith, K. A., Harden, E. A., Hartline, C.B., James, S.H., Kobzar, O., Kovalishyn, V., Vovk, A., Brovarets, V. In vitro activity of novel 4-iminohydantoin sulfamide derivatives against human cytomegalovirus. Chemical Papers. 2024, 1, 133-140. https://doi.org/10.1007/s11696-023-03038-1
6. Kovalishyn, V., Severin, O., Kachaeva, M., Kobzar, O., Keith, K. A., Harden, E. A., Caroll, H., James, S.H., Vovk, A., Brovarets, V. In silico design and experimental validation of novel oxazole derivatives against varicella zoster virus. Mol. Biotechnol. 2024, 66(4), 707-717. https://doi.org/10.1007/s12033-023-00670-w2020
7. Demchenko, S., Yarmoluk, S., Sukhovieiev, V., Golovchenko, O., Sukhovieiev, O., Demchenko, A. Syntheses and evaluation of novel 3-hydroxy-1,3-diaryl-2,3,5,6,7,8 hexahydroimidazo[1,2a]pyridine-1-ium bromides as potential anticancer agents. Pharmacia 2024, 71, 1-10. https://doi.org/3897/pharmacia.71.e135992
8. Tanchuk, V.Y., Kobzar, O.L., Vovk, A.I. Classification of active site conformations of protein tyrosine phosphatase 1B revisited. Bioorg. Acta 2024, 19(1), 54-60.
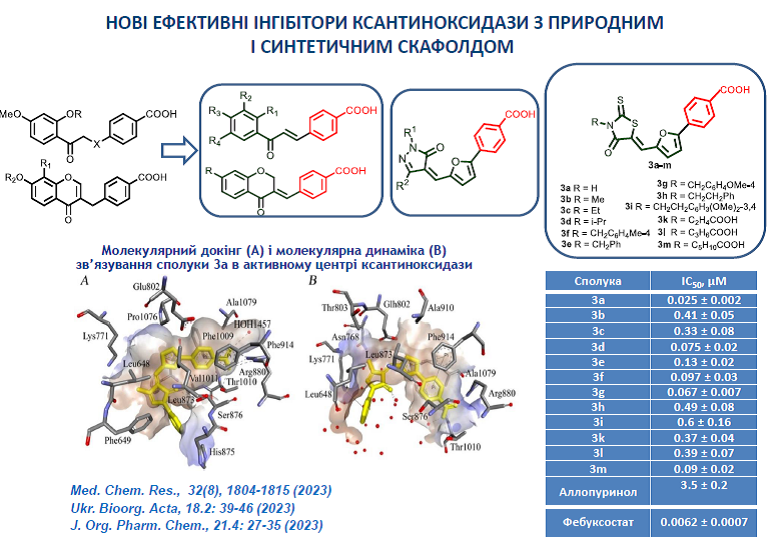
9. Beiko, A.V.; Kobzar, O.L.; Kachaeva, M.V.; Pilyo, S.G.; Tanchuk, V.Y.; Vovk, A.I. Inhibition of xanthine oxidase by pyrazolone derivatives bearing a 4-(furan-2-yl)benzoic acid moiety. J. Org. Pharm. Chem. 2023, 21, 27-35. https://doi.org/10.24959/ophcj.23.298726.
10 Beiko, A.V.; Kobzar, O.L.; Kachaeva, M.V.; Pilyo, S.G.; Kozachenko, O.P.; Vovk, A.I. Rhodanine-based 4-(furan-2-yl)benzoic acids as inhibitors of xanthine oxidase. Ukr. Bioorg. Acta 2023, 18(2), 31-40. https://doi.org/15407/bioorganica2023.02.031.
11. Los, O. V., Sinenko, V. O., Kobzar, O. L., Zhirnov, V. V., Vovk, A. I., Brovarets, V. S. Synthesis and in vitro anticancer potential of new thiazole-containing derivatives of rhodanine. Heterocycl. Compd. 2023, 59(6-7), 484-493. https://doi.org/10.1007/s10593-023-03220-z

12. Velihina, Y., Gesese, R., Zhirnov, V., Kobzar, O., Bui, B., Pilyo, S., Vovk, A., Shen, H., Brovarets, V. Design, synthesis and evaluation of the anti-breast cancer activity of 1, 3-oxazolo [4, 5-d] pyrimidine and 1, 3-oxazolo [5, 4-d] pyrimidine derivatives. RSC Med. Chem. 2023, 14(4), 692-699. https://doi.org/10.1039/D2MD00377E
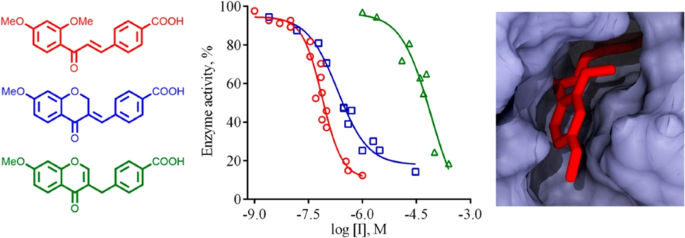
13. Kobzar, O. L., Tatarchuk, A. V., Mrug, G. P., Bondarenko, S. P., Demydchuk, B. A., Frasinyuk, M. S., Vovk, A. I. Carboxylated chalcones and related flavonoids as inhibitors of xanthine oxidase. Chem. Res. 2023, 32(8), 1804-1815. https://doi.org/10.1007/s00044-023-03109-8
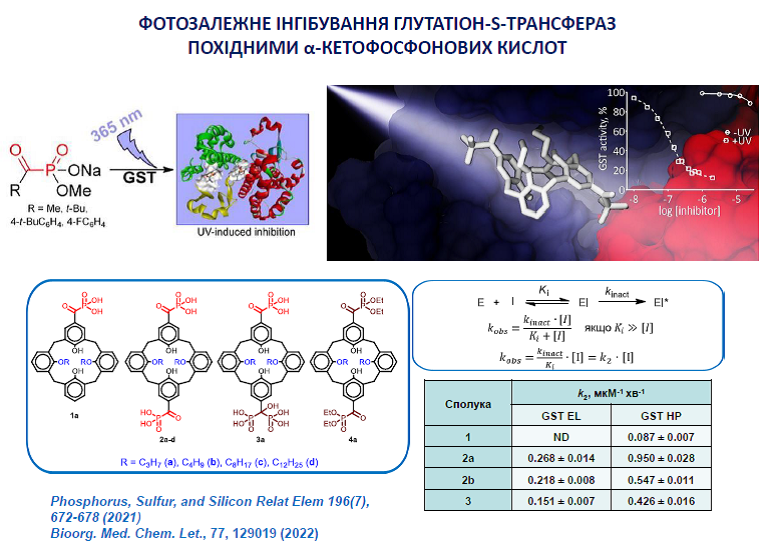
14. Kobzar O., Shulha Yu., Buldenko V., Cherenok S., Silenko O., Kalchenko V., Vovk A. Inhibition of glutathione S-transferases by photoactive calix[4]arene α-ketophosphonic acids. Med. Chem. Lett. 2022, 77, 129019. https://doi.org/10.1016/j.bmcl.2022.129019.

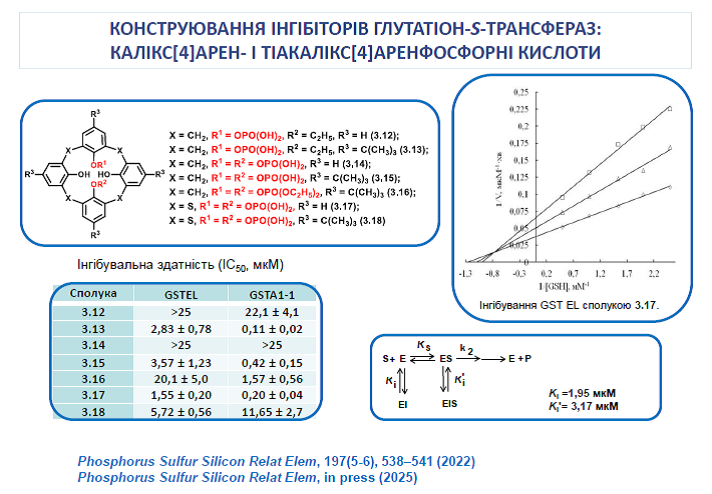
15. Silenko O., Cherenok S., Shulha, Kobzar O., Rusanov E., Karpichev E., Vovk A., Kalchenko V. Thiacalix[4]arene phosphoric acids. Synthesis, structure, and inhibition of glutathione S-transferases. Phosphorus, Sulfur, Silicon Relat. Elem. 2022, 197(5-6), 538-541. https://doi.org/10.1080/10426507.2021.2011877

16. Kobzar O. L., Shulha Yu. V., Buldenko V. M., Drapailo A. B., Kalchenko V. I., Vovk A. I. Inhibition of glutathione S-transferases by calix[4]arene-based phosphinic acids. Bioorg. Acta 2022, 17, 86-91. https://doi.org/10.15407/bioorganica2022.01.086
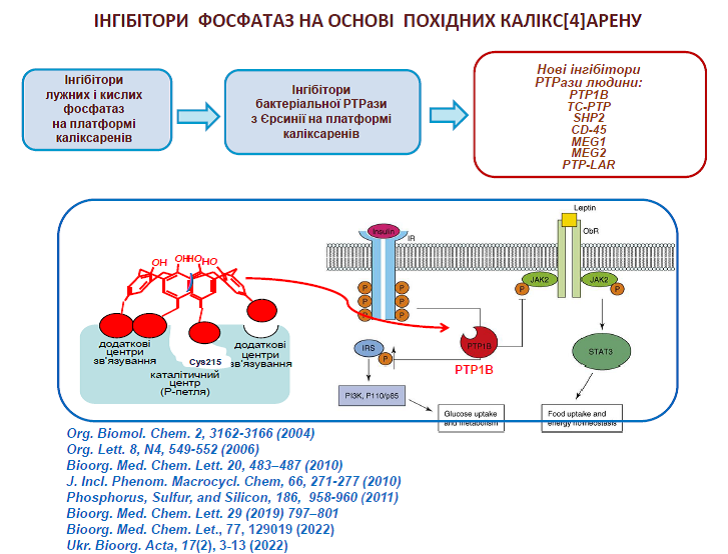
17. Kobzar, O. L., Cherenok, S. O., Kosterin, S., Kalchenko, V., Vovk, A. Biologically active calixarene phosphonic acids. Bioorg. Acta 2022, 17(2), 3-13. https://doi.org/10.15407/bioorganica2022.02.003
18. Velihina Y., Pilʹo S., Kobzar O., Zaliavska O., Prichard M. N., James S. H. Keith , Hartline C., Zhirnov V., Vovk A., Brovarets V. Synthesis of some oxazolo [4,5-d] pyrimidine derivatives and evaluation of their antiviral activity and cytotoxicity. Arkivok. 2022, 108-117. https://doi.org/10.24820/ark.5550190.p011.693
19. Kobzar, О. L., Shulha, Y. V., Buldenko, V. M., Mrug, G. P., Kolotylo, M. V., Stanko, O. V., Onysko P.P., Vovk, А. I. Alkyl and aryl α-ketophosphonate derivatives as photoactive compounds targeting glutathione S-transferases. Phosphorus, Sulfur, Silicon Relat. Elem. 2021, 196(7), 672-678. https://doi.org/10.1080/10426507.2021.1901703

20. Shatursky, O.Y., Manoilov, K.Y., Gorbatiuk, O.B., Usenko, M.O., Zhukova, D.A., Vovk, A.I., Kobzar, O.L., Trikash, I.O., Borisova. T.A., Kolibo, D.V., Komisarenko, S.V. The geometry of diphtheria toxoid CRM197 channel assessed by thiazolium salts and nonelectrolytes. Biophys J. 2021, 120(12), 2577-2591. https://doi.org/10.1016/j.bpj.2021.04.028.
21. А.І. Вовк, О.Л. Кобзар, Ю.М. Пархоменко. О-ацилзаміщені похідні і структурні аналоги вітаміну В1 . В кн. Синтез і біоактивність функціоналізованих азотовмісних гетероциклів / За редакцією А.І. Вовка. – Київ: ТОВ «НВП «Інтерсервіс», 2021. С. 151-180.
22. Sukhoveev, O. V., Burlaka, Y. B., Grin, N. V., Vovk, A. I., & Verevka, S. V. Studies of alterations of the cellular membrane barrier function at laryngeal cancer. Medical and Clinical Chemistry, 2021, 23(1), 5-12. https://doi.org/11603/mcch.2410-681X.2021.i1.12101
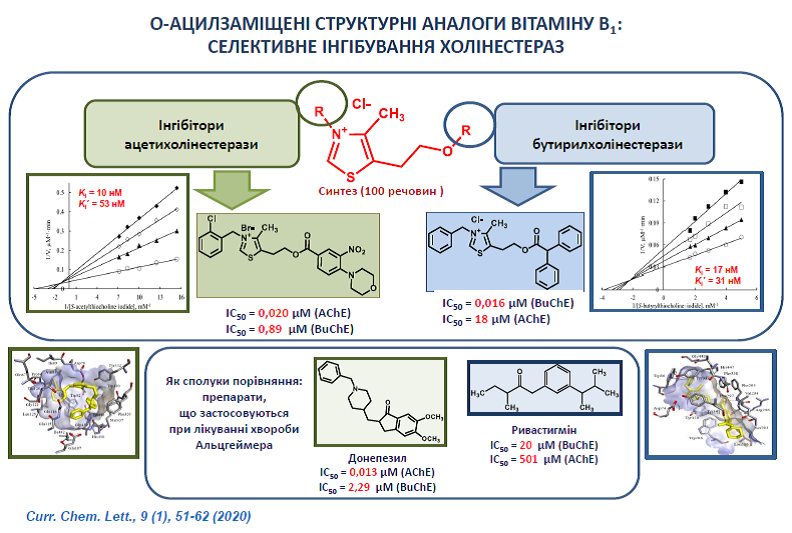
23. Kobzar O., Ocheretniuk A., Buldenko V., Babiy L., Kozachenko O., Brovarets V., Vovk A. O-Substituted N(3)-benzyl analogs of vitamin B1 as inhibitors of acetylcholinesterase or butyrylcholinesterase. Chem. Lett. 2020, 9(1), 51-62. http://doi.org/10.5267/j.ccl.2019.7.002
24. Muzychka, O. V., Kobzar, O. L., Shablykin, O. V., Brovarets, V. S., & Vovk, A. I. (2020). 5-Substituted N-(9H-purin-6-yl)-1, 2-oxazole-3-carboxamides as xanthine oxidase inhibitors. Bioorg. Acta 2020, 15(1), 20-25. https://doi.org/10.15407/bioorganica2020.01.020
25. Кобзар, О. Л., Година, Д. М., Синенко, В. О., Ковалішин, В. В., Трохименко, О. П., Сливчук, С. Р., Музичка, О. В. Прогнозування біоактивності і синтез нових 3-заміщених 5-тіазолілметиленроданінів. Доповіді НАН України. 2020, 5, 70-77. https://doi.org/10.15407/dopovidi05.070
26. Кобзар, О. Л., Татарчук, А. В., Качаєва, М. В., Пільо, С. Г., Суховєєв, О. В., Суховєєв, В. В., Вовк, А. І. Азометинові похідні п-амінобензойної кислоти як антиоксиданти та інгібітори ксантиноксидази. Доповіді НАН України. 2020, 6, 74-82. https://doi.org/10.15407/dopovidi2020.06.074
27. Kobzar O.L., Sinenko V.O., Shulha Y.V., Buldenko V.M., Hodyna D.M., Pilyo S.G., Brovarets V.S., Vovk A. I. Synthesis and evaluation of new thiazole-containing rhodanine-3-alkanoic acids as inhibitors of protein tyrosine phosphatases and glutathione S-transferases. Ukr. Bioorg. Acta 2020, 15(2), 33-40. https://doi.org/10.15407/bioorganica2020.02.033
28. Parkhomenko, Y., Vovk, A., & Protasova, Z. (2020). Vitamin B1 and the pyruvate dehydrogenase complex. In Molecular Nutrition (pp. 185-206) (Academic Press) https://doi.org/10.1016/B978-0-12-811907-5.00012-9
29. Poda G., Tanchuk V. "Computational Methods for the Discovery of Chemical Probes." In The Discovery and Utility of Chemical Probes in Target Discovery. 2020. 39-68. (Royal Society of Chemistry) https://doi.org/10.1039/9781839160745
30. Mezhenska O., Rebriev A., Kobzar O., Zlatoust N., Vovk A., Parkhomenko Yu. Non-coenzyme properties of thiamine: evaluation of binding affinity to malate dehydrogenase isoforms. Biotechnologia Acta 2020, 13(4), 26-38. https://doi.org/10.15407/biotech13.04.026
31. Shalimov, O., Rusanov, E., Muzychka, O., & Onys’ ko, P. (). Novel convenient approach to 6-, 7-, and 8-numbered nitrogen heterocycles incorporating endocyclic sulfonamide fragment. Molecules 2020, 25(12), 2887. https://doi.org/10.3390/molecules25122887
Публікації співробітників відділу за 2004-2019 рр.
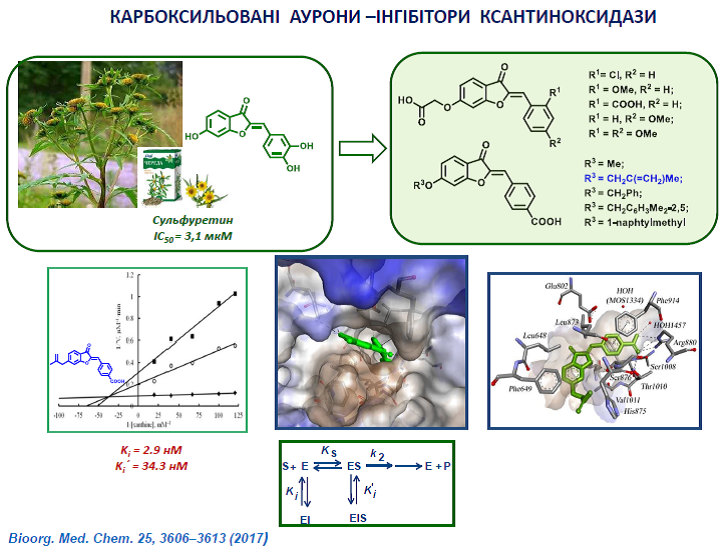
32. Muzychka O.V., Kobzar O.L., Popova A.V., Frasinyuk M.S., Vovk A.I. Carboxylated aurone derivatives as potent inhibitors of xanthine oxidase. Bioorg. Chem. 2017, 25, 3606–3613. https://doi.org/10.1016/j.bmc.2017.04.048

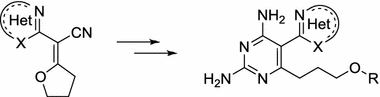
33. Khilya O., Milokhov D.S., Kononets L.A., Kobzar O.L., Vovk A.I., Volovenko Yu.M.. Synthesis and evaluation of new 2,6-diamino-5-hetarylpyrimidines as inhibitors of dihydrofolate reductase. Monatsh. Chem. 2018, 149, 813–822. DOI 10.1007/s00706-017-2032
34. Ocheretniuk A.D., Kobzar O.L., Mischenko I.M., Vovk I. N-Phenacylthiazolium alts as inhibitors of cholinesterases. French-Ukrainian J. Chem. 2017, 5, №2. https://doi.org/10.17721/fujcV5I2P1-14
35. Burlaka Y.B., Sukhoveev O.V., Grin N.V., Khilchevskyi O.M., Verevka S.V. EPR spectroscopy studies of changes in erythrocyte membranes in patients with laryngeal cancer. Experimental Oncology 2017, 39, 49–52. http://dspace.nbuv.gov.ua/handle/123456789/137612
36. Tanchuk V.Yu., Tanin V.O. Vovk A., Poda G. A New, Improved Hybrid Scoring Function for Molecular Docking and Scoring Based on AutoDock and AutoDock Vina. Chem. Biol. Drug Des. 2016, 87, 618-625. https://doi.org/10.1111/cbdd.12697
37. Melnyk, A. K., Sukhoveev, O. V., Kononets, L. A., Khilchevsky, O. M., Shulga, S. M., Kukhar, V. P., & Vovk, A. I. An EPR spin probe study of liposomes from sunflower and soybean phospholipids. Liposome Res. 2016, 26, 80-86. https://doi.org/10.3109/08982104.2015.1039031
38. Trush V.V., Kharchenko S.G., Tanchuk V.Yu., Kalchenko V.I., Vovk A.I. Phosphonate monoesters on a thiacalix[4]arene framework as potential inhibitors of protein tyrosine phosphatase 1B. Biomol. Chem. 2015, 13, 8803-8806. https://doi.org/10.1039/C5OB01247C
39. Trush V.V., Tanchuk V.Y., Cherenok S. O., Kalchenko V.I., Vovk A.. Evaluation of inhibition of protein tyrosine phosphatase 1B by calixarene-based α-ketophosphonic acids. Chem. Biol. Lett. 2015, 2, 1-5. https://pubs.iscience.in/journal/index.php/cbl/article/view/253
40. Kobzar O.L., Shevchuk M.V., Lyashenko A.N., Tanchuk V.Yu., Romanenko V.D., Kobelev S.M.,Averin A.D., Beletskaya I.P., Vovk A.I., Kukhar V.P. Phosphonate derivatives of tetraazamacrocycles as new inhibitors of protein tyrosine phosphatases. Org. Biomol. Chem. 2015, 13, 7437-7444. https://doi.org/10.1039/c5ob00713e
41. Kobzar O.L., Trush V.V., Tanchuk V.Yu., Vovk A.I. Inhibitory potential of polyhydroxylated fullerenes against protein tyrosine phosphatase 1B. Biochem. J. 2015, 87, 24-31. https://doi.org/10.15407/ubj87.04.024
42. Tanchuk V., Tanin V., Vovk A., Poda G. A new scoring function for molecular docking based on AutoDock and AutoDock Vina. Drug Discov. Technol. 2015, 12, 170-178. https://doi.org/10.2174/1570163812666150825110208
43. Mkrtchyan G., Aleshin V., Parkhomenko Y., Kaehne T., Di Salvo M. L., Parroni A., Contestabile R., Vovk A., Bettendorff L., Bunik V. Molecular mechanisms of the noncoenzyme action of thiamin in brain: biochemical, structural and pathway analysis Scientific Reports 2015, 5, Article number: 12583. https://doi.org/10.1038/srep12583
44. Kobzar O.L., Trush V.V., Tanchuk V.Yu., Zhilenkov A.V., Troshin P.A., Vovk A.I. Fullerene derivatives as a new class of inhibitors of protein tyrosine phosphatases. Med. Chem. Lett. 2014, 24, 3175–3179. https://doi.org/10.1016/j.bmcl.2014.04.110

45. Trush V.V., Tanchuk V.Y., Cherenok S.O., Kalchenko V.I., Vovk A.I. Calix[4]arene α-hydroxymethylphosphonic acids as potential inhibitors of protein tyrosine phosphatases. Org. Pharm. Chem. 2014, 1, 39-42. https://doi.org/10.24959/ophcj.14.782
46.Yu., Vovk A., Kalchenko V.I. Сalixarene methylene bisphosphonic acids as effectors of biochemical processes. In Book: “Ligands: synthesis, characterization and role in biotechnology”. Nova Science Publishers Inc. N.-Y., 2013. pp. 67-116.
47. Tanchuk V.Yu., Tanin V.O., Vovk A.I.. Analysis of сonformational flexibility of loop 110-120 of protein tyrosine phosphatase 1B. Biochim. J., 2013, 85, 73-80. http://dx.doi.org/10.15407/ubj85.05.073
48. Kalchenko V. I., Cherenok S.O., Kosterin S.O., Lugovskoy E.V., Komisarenko S.V., Vovk A.I., Tanchuk V.Y., Kononets L.A., Kukhar V.P. Calixarene phosphonous acids: synthesis and biological activity. Phosphorus, Sulfur, and Silicon 2013, 188, 232-237. https://doi.org/10.1080/10426507.2012.743147

49. Tanchuk V.Yu., Tanin V.O., Vovk A.I. QSAR models for predicting protein tyrosine phosphatase 1B inhibition by structurally diverse inhibitors. Org. Pharm. Chem. 2013, 11, 51-56.
50. Trush V.V., Cherenok S.O., Tanchuk V.Yu., Kukhar V.P., Kalchenko V.I., Vovk A.I. Calix[4]arene methylenebisphosphonic acids as inhibitors of protein tyrosine phosphatase 1B Med. Chem. Lett. 2013, 23, 5619–5623. https://doi.org/10.1016/j.bmcl.2013.08.040

51. Khakina E.A., Yurkova A.A., Peregudov A.A., Troyanov S.I., Trush V.V., Vovk A.I., Mumyatov A.V., Martynenko V.M., Balzarini J., Troshin P.A. Highly selective reactions of C60Cl6 with thiols for synthesis of functionalized [60]fullerene derivatives. Commun. 2012, 48, 7158-7160. https://doi.org/10.1039/C2CC32517A
52. Tanchuk V.Y., Tanin V.O., Vovk A.I. Classification of binding site conformations of protein tyrosine phosphatase 1B. Chem Biol Drug Des. 2012, 80, 121-128. https://doi.org/10.1111/j.1747-0285.2012.01370.x

53. Cherenok S.O., Yushchenko O.A., Tanchuk V.Yu., Mischenko I.M., Samus N.V., Ruban O.V., Matvieiev Y.I., Karpenko J.A., Kukhar V.P., Vovk A.I., Kalchenko V.I. Calix[4]arene-α-hydroxyphosphonic acids. Synthesis, stereochemistry, and inhibition of glutathione S-transferase. ARKIVOC 2012, 278 – 298. http://dx.doi.org/10.3998/ark.5550190.0013.421
54. Vovk A.I., Mischenko I.M., Cherenok S.O., Tanchuk V.Yu., Kalchenko V.I., Kukhar V.P. Phosphorylated calix[4]arenes as inhibitors of glutathione S-transferase. Phosphorus, Sulfur, and Silicon 2011, 186, 961-963. https://doi.org/10.1080/10426507.2010.514313
55. Vovk A.I., Tanchuk V.Yu., Kononets L.A., Cherenok S.O., Drapailo A.B., Kalchenko V.I., Kukhar V.P. A novel approach to the design of phosphonate inhibitors of protein tyrosine phosphatase. Phosphorus, Sulfur, and Silicon 2011, 186, 958-960. https://doi.org/10.1080/10426507.2010.521213
56. Kachkovskyi G.O., Kononets L.A., Tanchuk V.Yu., Vovk A.I., Kolodiazhnyi O.I. Synthesis and Evaluation of 1-Aryl-1-(7-carboxyisoindolin-1-one-2-yl)methyl-phosphonic Acid Derivatives as Inhibitors of Protein Tyrosine Phosphatase. Phosphorus, Sulfur, and Silicon 2011, 186, 956-957. https://doi.org/10.1080/10426507.2010.508063
57. Shapoval G.S., Babii L.V., Kruglyak O.S., VovkI. Antioxidant activity of thiamine and its structural analogs in reactions with electrochemically generated hydroxyl radicals and hydrogen peroxide. Theor. Exp. Chem. 2011, 47, 55-60. https://doi.org/10.1007/s11237-011-9185-y
58. Vovk A.I., Kononets L.A., Tanchuk V.Yu., Cherenok S.O., Drapailo A.B., Kalchenko V.I., Kukhar V.P. Inhibition of Yersinia protein tyrosine phosphatase by phosphonate derivatives of calixarenes. Med. Chem. Lett. 2010, 20, 483-487. https://doi.org/10.1016/j.bmcl.2009.11.126

59. Vovk A.I., Kononets L.A., Tanchuk V.Yu., Drapailo A.B., Kalchenko V.I., Kukhar V.P. Thiacalix[4]arene as molecular platform for design of alkaline phosphatase J. Incl. Phenom. Macrocycl. Chem. 2010, 66, 271-277. https://doi.org/10.1007/s10847-009-9607-9

60. Vovk A., Shivanyuk A.M., Bugas, R.V., Muzychka, O.V., Melnyk, A.K. Antioxidant and antiradical activities of resorcinarene tetranitroxides. Bioorg. Med. Chem. Lett. 2009, 19, 1314-1317. https://doi.org/10.1016/j.bmcl.2009.01.070

61. Vovk A.I., Mischenko I.M., Tanchuk V.Yu., Kachkovskii G.A., Sheiko S.Yu., Kolodyazhnyi O.I., Kukhar V.P. Stereoselectivity of binding of (N-benzylamino)benzylphosphonic acids to prostatic acid phosphatase. Med. Chem. Lett. 2008, 18, 4620–4623. https://doi.org/10.1016/j.bmcl.2008.07.021

62. Vovk A., Kalchenko V., Tanchuk V., Muzychka O., Muravyova I., Shivanyuk A., Cherenok S., Kukhar V. Сalixarene methylenebisphosphonic acids: alkaline phosphatase inhibition and docking studies. Phosphorus, Sulfur, and Silicon 2008, 183, 625-626. https://doi.org/10.1080/10426500701793311
63. Garazd M.M., Muzychka O.V., Vovk A.I., Nagorichna I.V., Ogorodniichuk A.S. Modified coumarins. 27. Synthesis and antioxidant activity of 3-substituted 5,7-dihydroxy-4-methylcoumarins. Nat. Comp. 2007, 43, 19-23.
https://doi.org/10.1007/s10600-007-0055-8
64. Cherenok S., Vovk A., Muravyova I., Shivanyuk A., Kukhar V., Lipkowski J., Kalchenko V. Calix[4]arene α-aminophosphonic acids: asymmetric synthesis and enantioselective inhibition of an alkaline phosphatase. Org. Lett. 2006, 8, 549-552. https://doi.org/10.1021/ol052469a

65. Vovk A.I., Kalchenko V.I., Cherenok S.A., Kukhar V.P., Muzychka O.V., Lozynsky M.O. Calix[4]arene methylenebisphosphonic acids as calf intestine alkaline phosphatase inhibitors. Org. Biomol. Chem. 2004, 2, 3162-3166. https://doi.org/10.1039/B409526J
Grants and competitive projects for the period 2019-2024
1. Project ‘Design, synthesis and activity of calixarene derivatives as inhibitors of protein tyrosine phosphatases and other enzymes’ under the National Research Fund of Ukraine competition ‘Support for research by leading and young scientists’. Organising body – Institute of Organic Chemistry of the National Academy of Sciences of Ukraine. Co-implementing organisation – V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences of Ukraine (2020-2023).
2. Scientific work ‘Creation of modern calixarene regulators of biochemical processes for medicine and biotechnology. Section 3. Search, design and establishment of properties of new potentially bioactive calixarene derivatives targeting therapeutically important protein targets’ State registration number 0124U001926 (Scientific and scientific-technical (experimental) works of the National Academy of Sciences of Ukraine in the priority area ‘Development of modern methods and technologies (in particular, molecular-genetic and biotechnological) to ensure the biological and food security of the state, creation of new drugs, methods and means of diagnosis for the needs of medicine and veterinary medicine ‘for 2023-2024).
3. A grant from the National Academy of Sciences to support research work by young scientists of the National Academy of Sciences of Ukraine ‘Prediction of bioactivity, synthesis and study of new thiazole-containing compounds as potential anti-cancer agents’ (2019-2020).
4. A grant from the National Academy of Sciences of Ukraine to research laboratories/groups of young scientists of the National Academy of Sciences of Ukraine for research in priority areas of science and technology development ‘Search for new photo-controlled inhibitors of therapeutically important enzymes’ (code: GR-KO-2020) (2020-2021).
5. Research project of young scientists of the National Academy of Sciences of Ukraine “New hydrazone-containing derivatives of nitrogenous heterocycles as potential inhibitors of purine metabolism”, state registration number 0121U111935 (2021-2022).
Defences of the dissertations of the Department researchers (since 2002)
L.V. Babiy, Dissertation for the degree of Candidate of Chemical Sciences ‘Model oxidative transformations of thiamine and thiamine phosphates’ – 2002.
O.V. Muzychka, Dissertation for the degree of Candidate of Chemical Sciences ‘Kinetic patterns of inhibition of alkaline phosphatase from calf intestines by L-cysteine reduced with glutathione and phosphonic acids’ – 2004.
R.V. Bugas, Dissertation for the degree of Candidate of Chemical Sciences ‘Modelling of vitamin B1 and its structural thiazole analogues transformations in biological transport processes’ – 2004.
L.A. Kononets, Dissertation for the degree of Candidate of Biological Sciences ‘Sulphur-containing derivatives of phosphonic acids as phosphatase inhibitors’ – 2012.
V.V. Trush, Dissertation for the degree of Candidate of Chemical Sciences ‘Inhibition of protein tyrosine phosphatase 1B by phosphonate derivatives of calix[4]arenes’ – 2015.
O.L. Kobzar, Dissertation for the degree of Candidate of Chemical Sciences ‘Potential inhibitors of protein tyrosine phosphatases based on tetraazamacrocycles and fullerenes’ – 2015.
V.O. Tannin, Dissertation for the degree of Candidate of Chemical Sciences ‘New approaches to in silico research of protein tyrosine phosphatase 1B inhibitors’ – 2016.
V.M. Buldenko, Dissertation for the degree of Candidate of Chemical Sciences (Doctor of Philosophy) ‘Sulfonilcalyx[4]arene as a platform for designing phosphatase inhibitors.’ – 2019.
A.D. Ocheretnyuk, Dissertation for the degree of Candidate of Chemical Sciences (Doctor of Philosophy) ‘Thiazolium salts as acetylcholinesterase inhibitors.’ – 2019.
Yu.V. Shulga, Dissertation for the degree of Doctor of Philosophy ‘Phosphonic, phosphinic and phosphoric acids as inhibitors of glutathione-S-transferases.’ – 2023.
A.V. Beiko, Dissertation for the degree of Doctor of Philosophy ‘Xanthine oxidase inhibitors based on carboxylated flavonoids and azole derivatives.’ – 2024.

Senior Researcher, Candidate of Chemical Sciences Oleksandr Kobzar

Junior Research Fellow, Doctor of Philosophy Yuriy Shulga

Researcher, Candidate of Chemical Sciences Alexander Sukhoveev

Junior Research Fellow, Doctor of Philosophy Alyona Beiko

In the cafe. Left – V. Buldenko, O. Kobzar, A. Vovk. Right – A. Ocheretniuk, O. Muzychka, I. Mishchenko, L. Kononets

O. Kobzar (foreground), A. Ocheretniuk and V. Buldenko (2018)

Postgraduate student V. Buldenko at work (2018)
Postgraduate student A. Ocheretniuk (2019)

V. Tanchuk (right) and V. Tanin (2016)

Y.V. Shulga – postgraduate student of the department (2019)

Department employees (2016) – V. Trush, O. Kobzar, O. Sukhoveev, L. Kononets, A. Vovk, O. Muzychka, V. Tanchuk, I. Mishchenko, A. Ocheretnyuk, V. Buldenko

Department employees in the laboratory (2010) – A. Vovk, L. Babiy, N. Samus, I. Mishchenko, O. Muzychka, L. Kononets, R. Bugas
V.P. Kukhar Institute
of Bioorganic Chemistry and Petrochemistry
NAS of Ukraine
© 2026 IBOPC NAS of Ukraine