The history of the department dates back to 1970, when a thematic group headed by a young Candidate of Chemical Sciences, Valery Kukhar, was formed within the Department of Organoelement Isocyanate Chemistry of the Institute of Organic Chemistry (Head of the Department: Academician of the Academy of Sciences of the Ukrainian SSR Academy, O.V. Kirsanov). In 1974, the abovementioned group expanded into a new Department of Chemistry of Polyhalogenated Organics from the discontinued Department of Synthesis of Growth-Activating Substances. In addition to chemists, the newly formed department included biologists who studied the physiological activity of synthesised compounds in plants. In 1983, by a resolution of the Presidium of the Academy of Sciences of the Ukrainian SSR, the Department of Bioorganic Chemistry of the Institute of Organic Chemistry was established, which included a research department that, after being renamed, received its current name, the Department of Fine Organic Synthesis. In 1986, the department was transferred to the newly established Institute of Bioorganic Chemistry of the Academy of Sciences of the Ukrainian SSR (since 1989, the Institute of Bioorganic Chemistry and Petrochemistry of the Academy of Sciences of the Ukrainian SSR ). From 1986 to 2017, the department was headed by an Academician of the National Academy of Sciences of Ukraine, Valery Kukhar. Since 2017, the Department of Fine Organic Synthesis has been headed by his student, Doctor of Chemical Sciences Ihor I. Gerus.
The primary scientific activity of the Department involves the development of methods for the targeted synthesis of phosphorus- and fluorine-containing isoelectronic and bioisosteric structural analogues of natural compounds. Scientists of the Department have found that the introduction of fluorine atoms into the structure of molecules does not lead to significant changes in the spatial structure of organic molecules since the sizes of hydrogen and fluorine atoms are very close, but the special electronic properties of the fluorine atom and fluorine-containing groups often lead to fundamental and positive changes in the chemical behaviour and biological activity of compounds. Another interesting and promising area of the Department's work is the synthesis and study of organophosphorus molecules containing fluorine fragments.
Main scientific and practical achievements of the Department
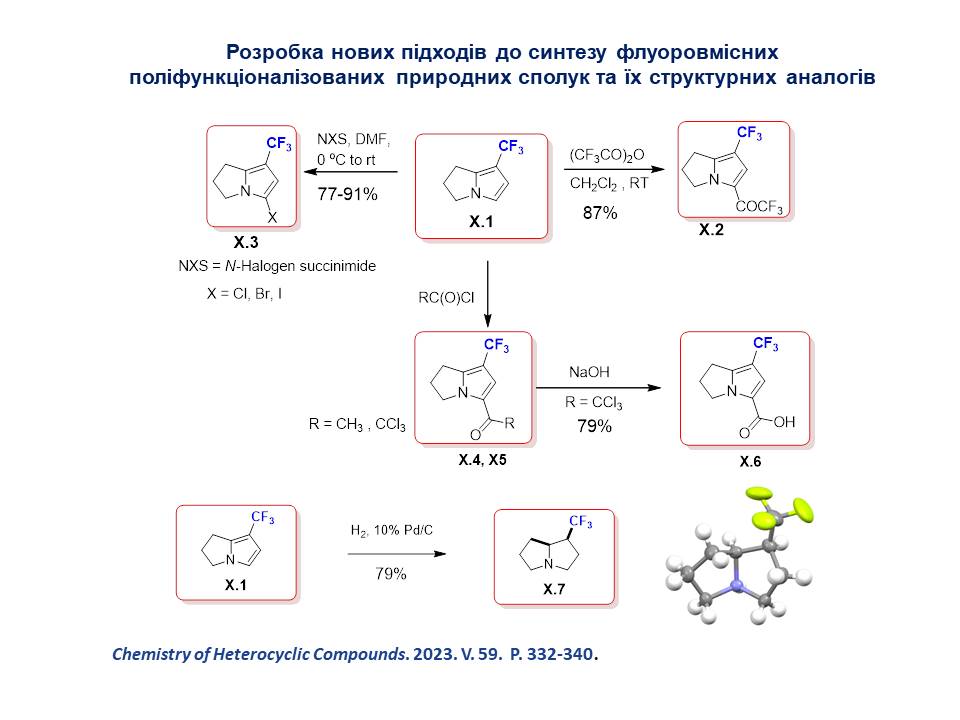
Methods for the synthesis of 7-(trifluoromethyl)-2,3-dihydro-1H-pyrrolizines and bicyclic trifluoromethyl-containing pyrroles, which are effective synthetic antimicrobial agents with a broad spectrum of action, including against multidrug-resistant gram-positive and gram-negative bacterial pathogens, have been developed.
Effective methods for the synthesis of fluorine-containing polyfunctionalised derivatives of tetrahydrofuran, pyrrole, tetrahydrothiophene, cyclopentane, bicyclo[3.1.0]hexane and spiro[3.3]heptane have been developed.
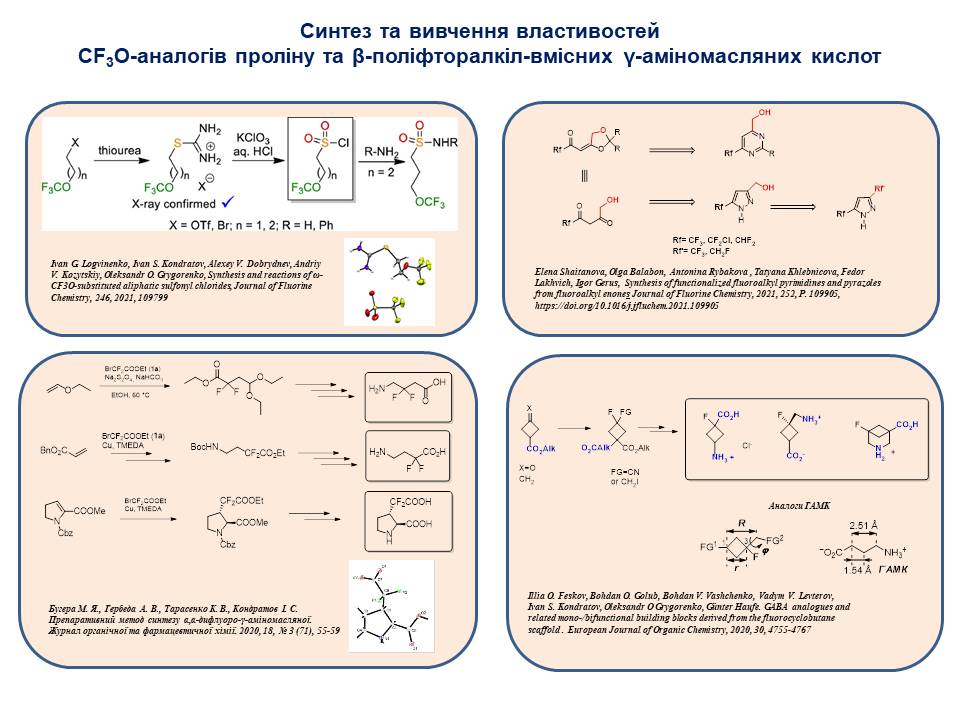
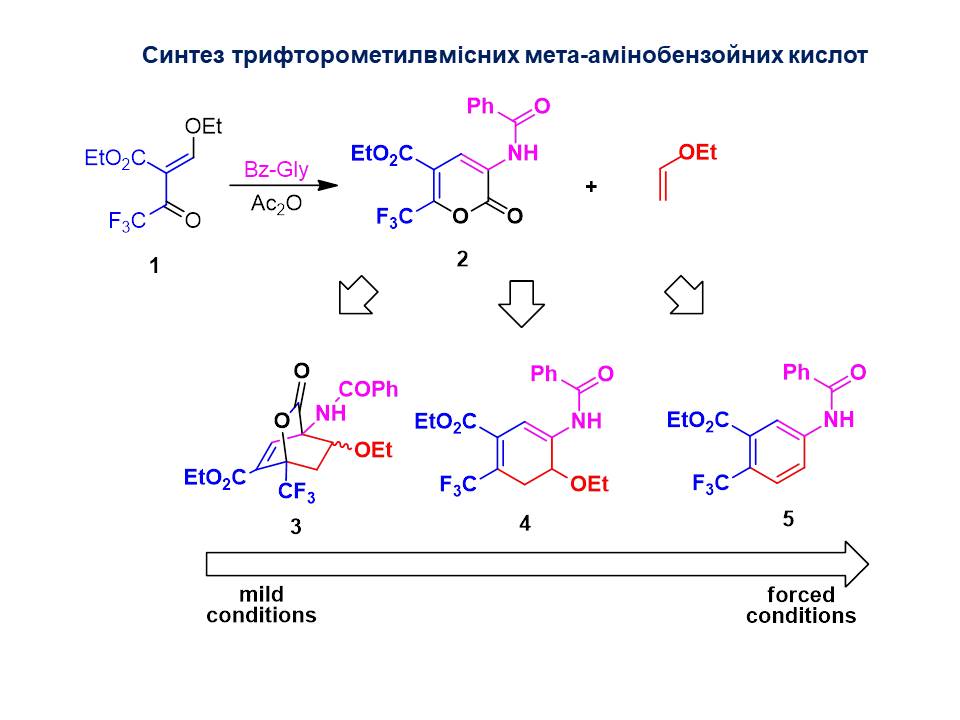
An efficient synthesis of ω-CF3O-substituted aliphatic sulfonyl chlorides has been developed. The deoxofluorination of aromatic and heteroaromatic acids with sulfur tetrafluoride was carried out to obtain trifluoromethyl-containing compounds that can be used as starting materials for the synthesis of new fluorinated amino acids, anilines and aliphatic amines. The reactions of trifluoromethyl-containing acrylonitrile and acrylate derivatives in [2+2], [3+2], [4+2] cycloaddition reactions, which are key to the synthesis of new trifluorinated amino acids, promising analogues of biologically active natural compounds, have been studied.
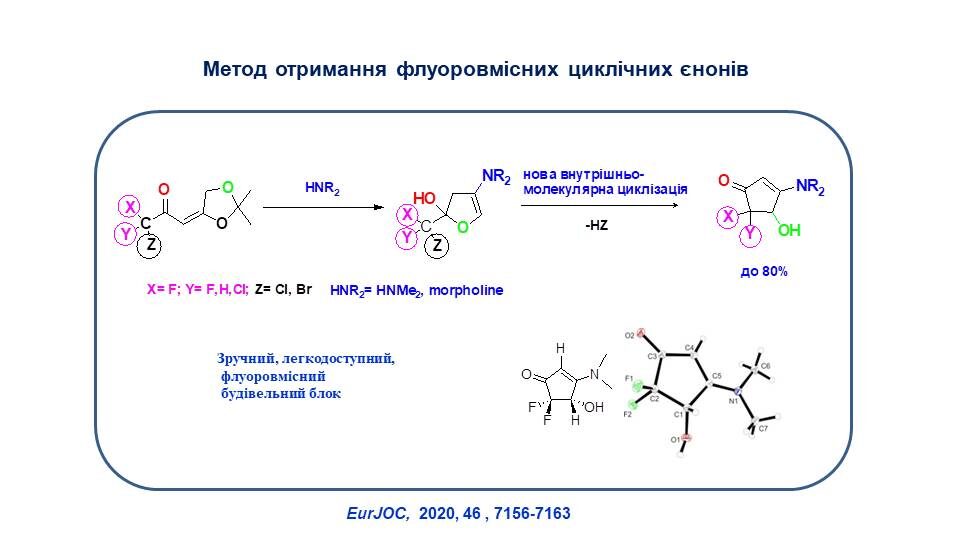
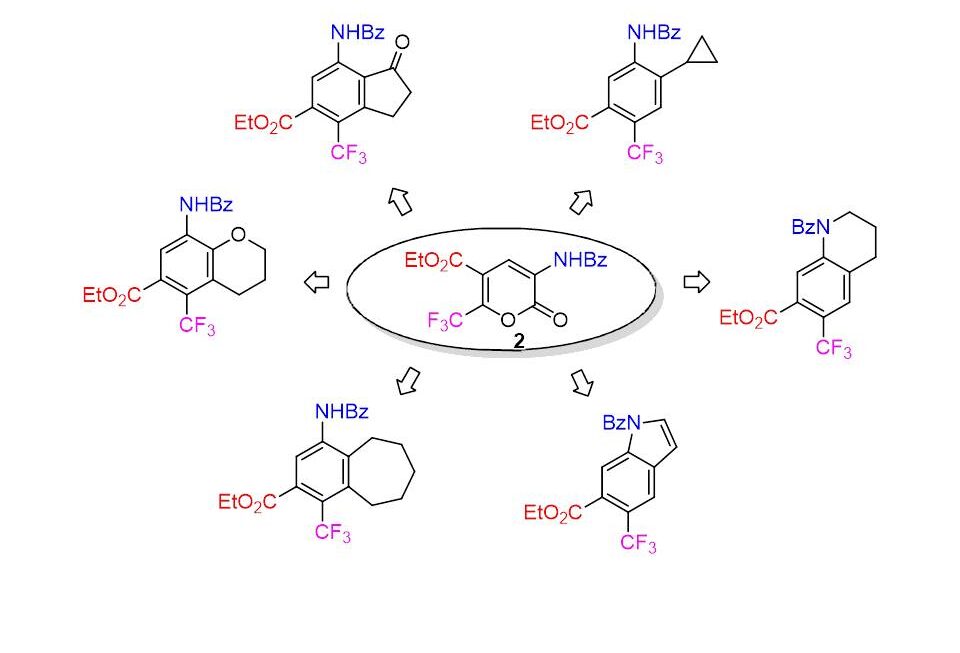
A method has been developed for the preparation of fluorinated cyclic enones, which are promising building blocks for the synthesis of various potentially bioactive compounds. The obtained enones react readily with an excess of secondary amines to form intramolecular electrocyclization products, cyclopentenones, the structure of which has been proved by NMR spectroscopy and X-ray crystallography.
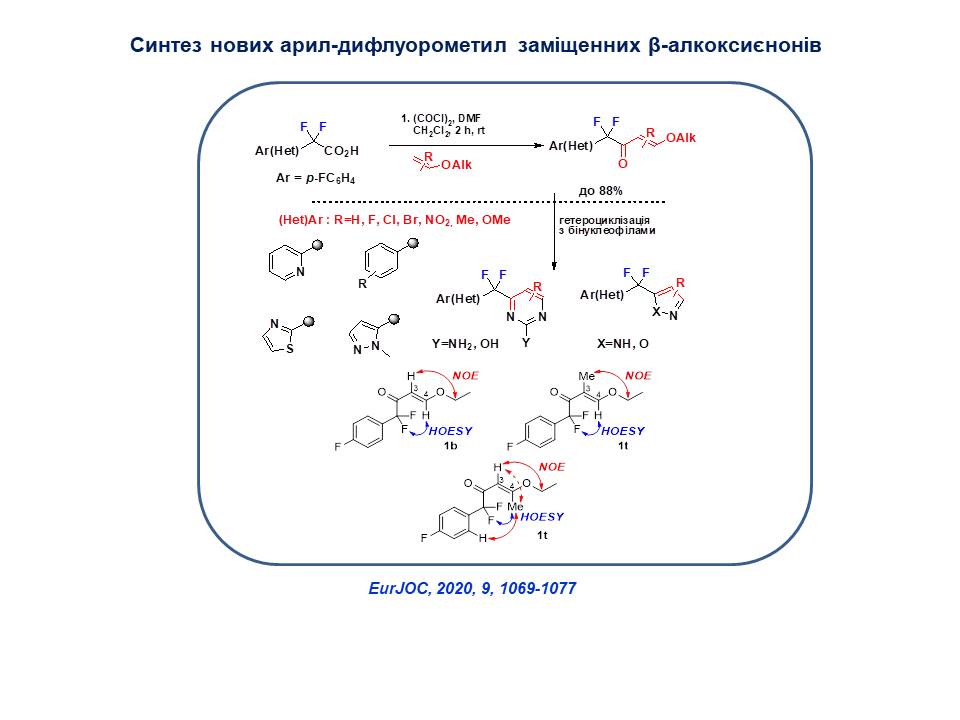
A convenient method for the synthesis of new aryl-difluoromethyl substituted β-alkoxyenones was proposed, which were subsequently used in the synthesis of a number of potentially biologically active heterocycles: pyrazoles, isoxazoles and pyrimidines with aryl(heteraryl)difluoromethylene substituents.
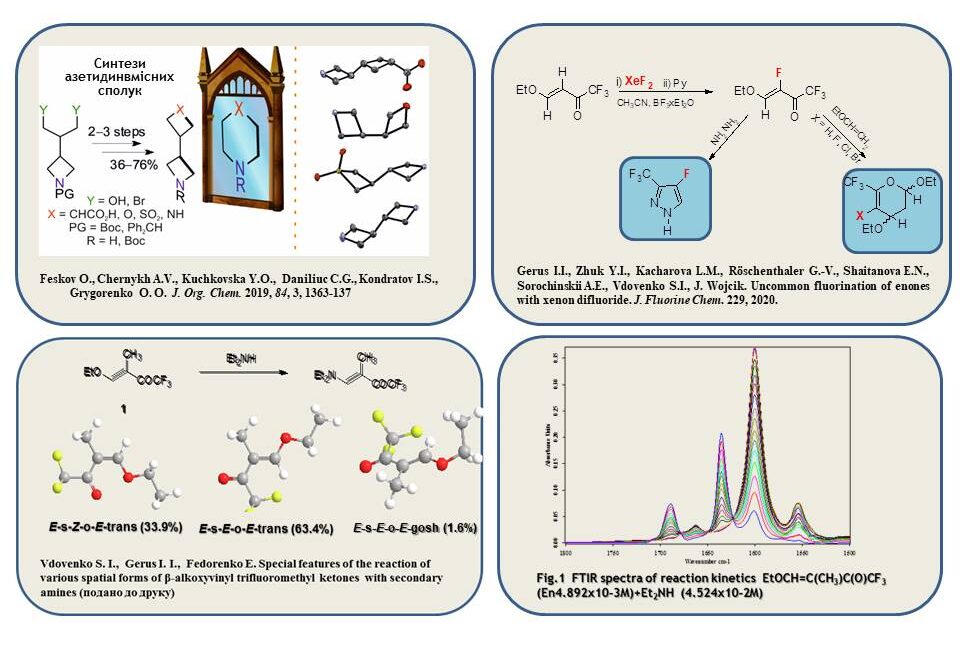
A convenient method for the synthesis of azithidine-containing compounds, which can be regarded as ‘stretched’ bioisosteres of six-membered heterocycles by their structural properties, has been developed.
The fluorination of readily available β-alkoxy-enones was developed using commercial reagents XeF2 and BF3 Et2O (as a catalyst), and new α-fluoro-β-alkoxy-enones were obtained. The synthesised compounds were used for the preparation of new fluorinated dihydropyrans and pyrazoles and are promising precursors in the synthesis of fluorinated sugars
A detailed study of the kinetics of the reactions of different spatial forms of β-substituted alkoxyvinyltrifluoromethyl ketones with secondary amines was conducted on the example of the reaction of different enone conformers 4-ethoxy-1,1,1-trifluoro-3-methylbut-3-en-2-one with diethyl amine. It is shown that this enone exists in three main conformers in solutions: E-s-E-o-E-cis and E-s-Z-o-E-cis and E-s-E-o-E-gosh.
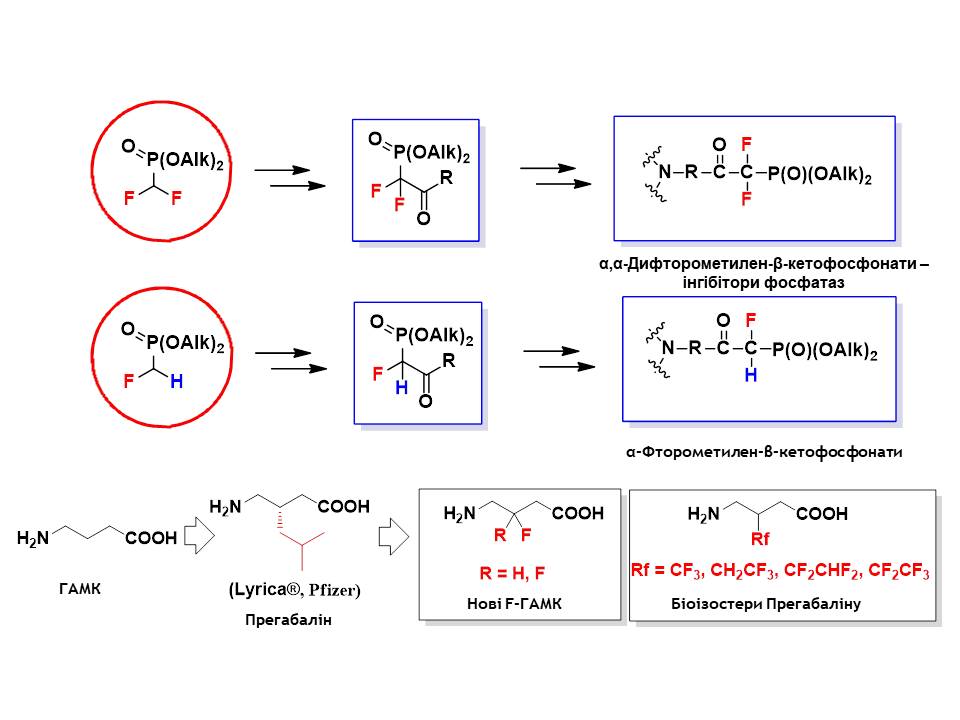
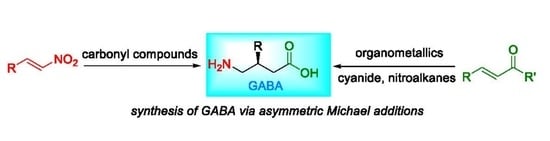
A series of fluorinated analogues of γ-aminobutyric acid GABA (the most important neurotransmitter of the human central nervous system), which are isosters of the existing commercial drug Pregabalin, were synthesised.
Scientists of the Department

Leading Research Fellow, Doctor of Chemical Sciences, Professor, Vladyslav Demydovych Romanenko
https://orcid.org/0000-0001-6642-5413
https://www.scopus.com/authid/detail.uri?authorId=7102244594

Senior Research Fellow, Candidate of Chemical Sciences, Serhiy Ivanovych Vdovenko
https://www.scopus.com/authid/detail.uri?authorId=8603681600
https://orcid.org/0000-0002-0988-5250

Senior Research Fellow, Candidate of Chemical Sciences, Karen Volodymyrovych Tarasenko
https://www.scopus.com/authid/detail.uri?authorId=21935229100&origin=recordpage
https://orcid.org/0000-0003-2147-8399

1 Category Engineer, Candidate of Chemical Sciences, Oleksandr Yevheniyovych Sorochinsky
https://www.scopus.com/authid/detail.uri?authorId=6602509268
https://orcid.org/0000-0002-0132-2664

1 Category Engineer, Candidate of Chemical Sciences, Ivan Serhiyovych Kondratov
https://www.scopus.com/authid/detail.uri?authorId=8939218400
https://orcid.org/0000-0003-0192-0725

Junior Research Fellow, Candidate of Chemical Sciences, Olexandr Mykolayovych Khilchevsky
https://www.scopus.com/authid/detail.uri?authorId=6508302225

1 Category Engineer, Candidate of Chemical Sciences, Olena Mykolayivna Shaitanova
https://www.scopus.com/authid/detail.uri?authorId=15923648500
https://orcid.org/0000-0001-7347-0116

Junior Research Fellow, Candidate of Chemical Sciences, Iryna Bronislavivna Kulyk

Junior Research Fellow, Candidate of Chemical Sciences, Violetta Henadiyivna Dolovanyuk
https://www.scopus.com/authid/detail.uri?authorId=35086062500
https://orcid.org/0000-0002-4324-1379

Junior Research Fellow, PhD, Anton Anatoliyovych Klipkov
https://www.scopus.com/authid/detail.uri?authorId=57214796718
https://orcid.org/0000-0002-2553-8232

Junior Research Fellow, Candidate of Chemical Sciences, Illia Oleksandrovych Feskov
https://www.scopus.com/authid/detail.uri?authorId=57056518700

1 Category Engineer, Candidate of Chemical Sciences, Maksym Yaroslavovych Bugera
https://www.scopus.com/authid/detail.uri?authorId=55925771000
https://orcid.org/0000-0003-2260-1379

1 Category Engineer, Olena Andriyivna Fedorenko
https://orcid.org/0009-0005-7067-5817

1 Category Engineer, Antonina Viktorivna Rybakova
https://www.scopus.com/authid/detail.uri?authorId=6701384263

2 Category Engineer, Yevheniya Volodymyrivna Ruchko
1 Category Engineer, Ivan Henadiyovych Lohvynenko
https://www.scopus.com/authid/detail.uri?authorId=57192995717
https://orcid.org/0000-0002-9367-1748
1 Category Engineer, Volodymyr Andriyovych Ahunovych
https://www.scopus.com/authid/detail.uri?authorId=57250602000
The most important publications of the Department researchers
1. Romanenko V.D. Organophosphorus Synthesis beyond P-Cl bond: The Development of Shelf-Stable Reagents for [RP] Transfer. Chem 2024. V. 28, N 19. Р. 1483-1512. DOI: 10.2174/0113852728323258240613061150

2. S.I. Vdovenko, I.I. Gerus, M. Pagacz-Kostrzewa, M. Wierzejewska. Comparison of kinetic and thermodynamic parameters of reaction of individual conformers of α‑substituted β‑ethoxyvinyl trifluoromethyl ketones with secondary amines. Reaction Kinetics, Mechanisms and Catalysis. 2024. V. 137, N 2. Р. 1-18. DOI:10.1007/s11144-024-02578-1
3. Q. Wang, Y. Bian, G. Dhawan, W. Zhang, A.E. Sorochinsky, A. Makarem, A. Soloshonok, J. Han. Fluorine-containing drugs approved by the FDA in 2023. Chinese Chemical Letters. 2024. V. 35. Р. 109780. doi: 10.1016/j.cclet.2024.109780

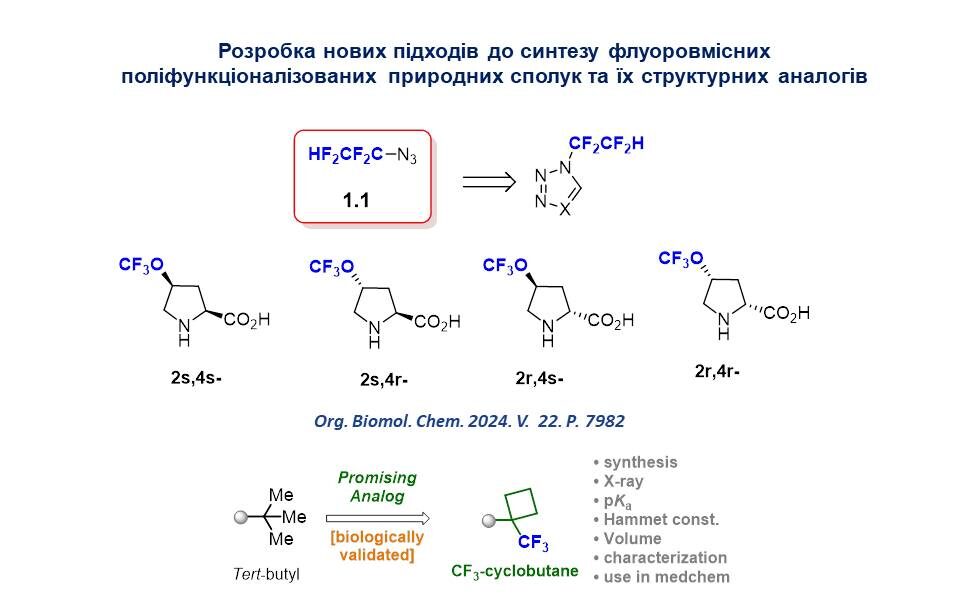
4. Logvinenko I.G., Sadkova I.V., Tolmachova N.A., Shishkina S.V., Daniliuc K.G.,Haufe G., Kondratov I.S. 4-Trifluoromethoxy proline: synthesis of stereoisomers and lipophilicity study. Org. Biomol. Chem. 2024. V. 22. Р. 7982-7988. DOI: 10.1039/D4OB00688G
5. Pahl A., Grygorenko O.O., Kondratov I.S., Waldmann H. Identification of readily available pseudo-natural products. RSC Med. Chem. 2024. V. 15. Р. 2709-2717. DOI: 10.1039/D4MD00310A
6. F. Liu, A.L. Kaplan, J. Levring, J. Einsiedel, S. Tiedt, K. Distler, N.S. Omattage, I.S. Kondratov, Y.S. Moroz, H.L. Pietz, J.J. Irwin, P. Gmeiner, B.K. Shoichet, J. Chen. Structure-based discovery of CFTR potentiators and inhibitors. Cell. 2024. V. 187, N 14. P. 3712-3725. doi: 10.1016/j.cell.2024.04.046

7. P. Janssen, F. Becker, F.T. Füsser, N. Tolmachova, T. Matviiuk, I. Kondratov, M.S. Weiss, D. Kümmel, O. Koch. Design and Crystallographic Screening of a Highly Sociable and Diverse Fragment Library Towards Novel Antituberculotic Drugs. ChemRxiv. 2024. doi: 10.26434/chemrxiv-2024-rpst3-v2
8. A.A. Klipkov, I.I. Gerus. Electrophilic reactions of 7-(trifluoromethyl)-2,3-dihydro-1H-pyrrolizine – the way towards new building blocks. J. Org. Pharm. Chem. 2023. V. 21, N 2. Р. 36-40. https://doi.org/10.24959/ophcj.23.284640
9. Logvinenko I., Dolovanyuk V.,Kondratov I. Multigram synthesis of 2-(trifluoromethoxy)ethan-1-ol and 3-(trifluoromethoxy)propan-1-ol – perspective Building Blocks for Drug Discovery. Ukrainica Bioorganica Acta. 2023. V. 18, N 1. Р. 27-31. https://doi.org/10.15407/bioorganica2023.01.027.
10. N.V. Lyutenko, A.E. Sorochinsky, V.A. Soloshonok. Asymmetric synthesis of pyroglutamic acids via Ni(II)-complex methodology. Chemistry of Heterocyclic Compounds. 2023. V. 59. Р. 332-340. DOI: 10.1007/s10593-023-03203-0

11. Romanenko V.D. Synthetic strategies toward and around the CF3S(O) structural motif. Current Organic Chemistry. 2023. V. 27. Р.411-434. DOI: 10.2174/1385272827666230517114921
12. Holovach S.,Melnykov K.P.,Poroshyn I.,Iminov R.T.,Dudenko D.,Kondratov I.S.,Levin M.,Grygorenko O.O. C−C Coupling through Nitrogen Deletion: Application to Library Synthesis. Chemistry A European Journal. 2023. V. 29, N 4.Р. e202203470. https://doi.org/10.1002/chem.202203470

13. Fink E. A., Bardine C., Gahbauer S., Singh I.Detomasi T. C., White K., Gu S.,Wan X., Chen J., Ary B., Glenn I., O’Connell J., O’Donnell H.,Fajtov P., Lyu J.,Vigneron S.,Young N.J.,Kondratov I.S.,Alisoltani A.,Simons L.M.,Lorenzo-Redondo R., Ozer E. A.,Hultquist J.F.,O’Donoghue A. J.,Moroz Y.S.,Taunton J.,Renslo A.R., Irwin J.J.,García-Sastre A.,Shoichet B.K.,Craik C.S. Large library docking for novel SARS‐CoV‐2 main protease non‐covalent and covalent inhibitors. Protein Science. 2023. V. 32. Р. e4712. https://doi.org/10.1002/pro.4712
14. Gahbauer S.,DeLeon C.,Braz J.M.,Craik V.,Kang H.J.,Wan X.,Huang X.-P., Billesbølle C.B.,Liu Y.,Che T.,Deshpande I.,Jewell M.,Fink E.A.,Kondratov I.S., Moroz Y.S.,Irwin J.J.,Basbaum A.I.,Roth B.L.,Shoichet B.K. Docking for EP4R antagonists active against inflammatory pain. Nature Communications. 2023. V. 14, N 1. Р. 8067. https://doi.org/10.1038/s41467-023-43506-6
15. S.I. Vdovenko,I.I. Gerus,M. Pagacz-Kostrzewa,M. Wierzejewska. Influence of the features of the spatial and electronic structure of α-substituted β-ethoxyvinyl trifluoromethyl ketones and secondary amines on their reactivity. Journal of Molecular Structure. 2022. V. 1255. Р. 132417. Doi: 10.1016/j.molstruc.2022.132417

16. J. Liu, W. Lin,A.E. Sorochinsky,G. Butler,A. Landa, J. Han,V.A. Soloshonok. Successful trifluoromethoxy-containing pharmaceuticals and agrochemicals. Journal of Fluorine Chemistry. 2022. V. 257-258. Р. 109978. doi.org/10.1016/j.jfluchem.2022.109978
17. J. Han, J. Escorihuela, S. Fustero,A. Landa,V.A. Soloshonok, A. Sorochinsky. Asymmetric Michael addition in synthesis of β-substituted GABA derivatives. Molecules. 2022. V. 27. Р. 3797. doi: 10.3390/molecules27123797.м
18. Q. Wang, J. Han, A. Sorochinsky, A. Landa, G. Butler, V. A. Soloshonok. The latest FDA-approved pharmaceuticals containing fragments of tailor-made amino acids and fluorine. Pharmaceuticals. 2022. V. 15. Р. 999. DOI:10.3390/ph15080999

19. V.D. Romanenko. From elusive monomeric metaphosphates to oligomeric metaphosphate reagents: New avenue to halogen-free phosphorylation of biomolecules. Current Organic Chemistry. 2022. V. 26, Р. 432-437. DOI: 10.2174/1385272826666220330111824
20. Chernykh A.V., Aloshyn D. ,Kuchkovska Yu.O., Daniliuc C.G.,Tolmachova N.A., Kondratov I.S., Zozulya S., Grygorenko O.O., Haufe G. Impact of β-perfluoroalkyl substitution of proline on the proteolytic stability of its peptide derivatives. Org. Biomol. Chem. 2022. V. 20. Р. 9337-9350. https://doi.org/10.1039/D2OB01430K
21. Logvinenko I.G.,Kondratov I.S.,Pridma S.O.,Tolmachova N.A.,Morev R.N., Dolovanyuk V.G.,Boretsky A.L.,Stepaniuk R.O.,Trofymchuk S.A., Mück-Lichtenfeld C., Daniliuc C.G.,Haufe G. Synthesis and physical chemical properties of CF3O-containg secondary amines - Perspective building blocks for drug discovery. Journal of Fluorine Chemistry. 2022. V. 257–258. Р. 109990. https://doi.org/10.1016/j.jfluchem.2022.109990

22. Homon A.A.,Shynder L.V.,Demchuk O.P.,Hryshchuk O.V.,Kondratov I.S.,Gerus I.I., Grygorenko O.O. Synthesis of 1,3-bifunctional cyclobutane derivatives with α-CHF2/CF3 group – advanced building blocks for medicinal chemistry. Journal of Fluorine Chemistry. 2022. V. 263. Р. 110041. https://doi.org/10.1016/j.jfluchem.2022.110041

23. Kondratov I.S.,Moroz Yu.S.,Irwin J.J.,Shoichet B.K. Drug building blocks and libraries at risk in Ukraine. Science. 2022. V. 376(6596). Р. 929. https://doi.org/10.1126/science.abq7841
24. Kondratov I.S.,Moroz Yu.S.,Grygorenko O.O.,Tolmachev A.A. The Ukrainian Factor in Early-Stage Drug Discovery in the Context of Russian Invasion: The Case of Enamine Ltd. ACS Med. Chem. Lett. 2022. V. 13. N 7. Р. 992-996. https://doi.org/10.1021/acsmedchemlett.2c00211

25. V.D. Romanenko, J.-M. Sotiropoulos. Six-membered rings with two or more heteroatoms with at least one boron. Comprehensive Heterocyclic Chemistry IV, Elsevier. New York. 2021. Р. 1-40. DOI: 10.1016/B978-0-12-818655-8.00098-6.
26. I.G. Logvinenko,.S. Kondratov,A.V. Dobrydnev,A.V. Kozytskiy,O.O. Grygorenko. Synthesis and reactions of ω-CF3O-substituted aliphatic sulfonyl chlorides. Journal of Fluorine Chemistry. 2021. V. 246. Р. 109799. doi.org/10.1016/j.jfluchem.2021.109799

27. J. Han,N. Lyutenko,A. Sorochinsky,A. Okawara,H. Konno,S. White,V. Soloshonok. Tailor-Made Amino Acids in Pharmaceutical Industry: Synthetic Approaches to aza-tryptophane derivatives. Chem. Eur. J. 2021. doi.org/10.1002/chem.202102485

28. E.N. Shaitanova, O.A. Balabon,A.N. Rybakova,T.S. Khlebnicova, F.А. Lakhvich, I.I. Gerus. Synthesis of functionalized fluoroalkyl pyrimidines and pyrazoles from fluoroalkyl enones. Journal of Fluorine Chemistry.2021. V. 252.Р. 109905. doi.org/10.1016/j.jfluchem.2021.109905

29. A.A. Homon, O.V. Hryshchuk, O.V. Mykhailenko, B.V. Vashchenko, K.P. Melnykov, O.M. Michurin, C.G. Daniliuc, I.I. Gerus, V.O. Kovtunenko, I.S. Kondratov, O. Grygorenko. 4-(Di‐/Trifluoromethyl)‐2‐heterabicyclo[2.1. 1]- hexanes: advanced fluorinated phenyl isosteres and proline analogues. European Journal of Organic Chemistry. 2021. https://doi.org/10.1002/ejoc.202100414

30. S. Trofymchuk,M. Bugera,A. Klipkov, V. Ahunovych,B. Razhyk,S. Semenov, A. Boretskyi, K. Tarasenko,P. Mykhailiuk. Scalable Approach to Fluorinated Heterocycles with Sulfur Tetrafluoride (SF4). J. Org. Chem. 2021.V. 86, № 17. Р. 12181-12198. https://doi.org/10.1021/acs.joc.1c01518

31. V.D. Romanenko. New trends in development of P-C bond forming reactions. Current Organic Chemistry. 2021. V.25, № 17. Р. 1937-1976. DOI: 10.2174/1385272825666210610153954
32. Romanenko V.D. Rings containing group 15 elements. Ref. Mod. Chem. Molecular Sciences. Elsevier. 2020. Р. 1-3.
33. Logvinenko I.G., Markushyna Y., Kondratov I.S.,Vashchenko B.V., Kliachyna M., Tokaryeva Yu., Pivnytska V.,Grygorenko O. O., Haufe G. Synthesis, physico-chemical properties and microsomal stability of compounds bearing aliphatic trifluoromethoxy group. Journal of Fluorine Chemistry. 2020. V. 231. Р. 109461. https://doi.org/10.1016/j.jfluchem.2020.109461
34. Bugera M.Ya,Tarasenko K.V.,Kondratov I.S.,Gerus I. I,,Vashchenko B.V., Ivasyshyn V.E.Grygorenko O.O. (Het)aryl difluoromethyl-substituted β-alkoxyenones: synthesis and heterocyclizations, European Journal of Organic Chemistry. 2020. V. 9. Р. 1069-1077. https://doi.org/10.1002/ejoc.201901833

35. Malashchuk A., Chernykh, A.V., Hurmach V.V., Platonov M.O., Onopchenko O., Zozulya S., Daniliuc C.G., Dobrydnev A.V., Kondratov I.S., Moroz Yu.S., Grygorenko O.O. Synthesis, biological evaluation, and modeling studies of 1,3-disubstituted cyclobutane-containing analogs of combretastatin A4, Journal of Molecular Structure. 2020. V. 1210. Р. 128025-128033. DOI: 10.1016/j.molstruc.2020.128025

36. Stepaniuk O.O.,Vashchenko B.V., Matvienko V.O.,Kondratov I.S.,Tolmachev A.A., Grygorenko O.O. Reactions of cyclic β-alkoxyvinyl α-keto esters with heteroaromatic NCC-binucleophiles. Chemistry of Heterocyclic Compounds. 2020. V. 56, № 3. Р. 377-385. https://doi.org/10.1007/s10593-020-02670-z
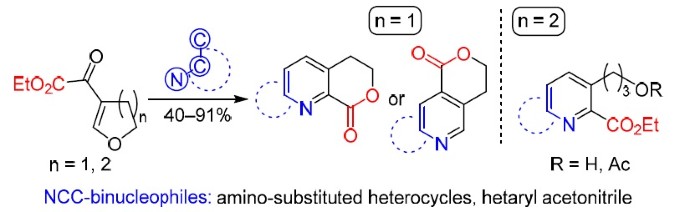
37. Stepaniuk O.O,Rudenko T.V.,Vashchenko B.V.,Matvienko V.O.,Kondratov I.S., Tolmachev A.A.,Grygorenko O.O. Synthesis of Fused Pyridine Carboxylates by Reaction of β-Alkoxyvinyl Glyoxylates with Amino Heterocycles, Synthesis. 2020. V. 52, № 13. Р. 1915-1926. DOI: 10.1055/s-0039-1707987

38. Feskov I.O., Golub B.O., Vashchenko B.V, Levterov V. V., Kondratov I.S., Grygorenko O.O., Haufe G. GABA Analogues and Related Mono‐/Bifunctional Building Blocks Derived from the Fluorocyclobutane Scaffold, European Journal of Organic Chemistry. 2020. V. 30. Р. 4755-4767. DOI: 10.1002/ejoc.202000733

39. Klipkov A.A., Sorochinsky A.E., Tarasenko K.V., Rusanova J.A.,Gerus I.I. Synthesis of trifluoromethyl and trifluoroacetyl substituted dihydropyrrolizines and tetrahydroindolizines, Tetrahedron Lett. 2020. V. 61. P. 151633. DOI:10.1016/j.tetlet.2020.151633

40. Gerus I.I, Zhuk Y.I, Kacharova L.M, Röschenthaler G., Shaitanova E.N., Sorochinskii A.E., Vdovenko S. I., Wojcik Y. Uncommon fluorination of enones with xenon difluoride, Journal of Fluorine Chemistry. 2020. V. 229. Р. 109413. DOI:10.1016/j.jfluchem.2019.109413

41. Trofymchuk S., Bugera M., Klipkov A., Razhyk B.,Semenov S.,Tarasenko K., Starova V., Zaporozhets O., Tananaiko O., Alekseenko A., Pustovit Y.,Kiriakov O., Gerus I.,Tolmachev A., Mykhailiuk P. Deoxofluorination of (Hetero) aromatic Acids, The Journal of Organic Chemistry. 2020. V. 85, № 5.Р. 3110-3124. DOI:10.1021/acs.joc.9b03011

42. Trofymchuk S.A, Kliukovskyi D.V., Semenov S.V., Khairulin A.R., Shevchenko V.O., Bugera M.Y., Tarasenko K.V., Volochnyuk D.M., Ryabukhin S.V. Semi-Industrial Fluorination of β-Keto Esters with SF4: Safety vs Efficacy. Synlett. 2020. V. 31, № 06. Р. 565-574. DOI: 10.1055/s-0037-1610744

Defences of the dissertations of the Department researchers (since 2002)
I.B. Kulyk, Dissertation for the degree of Candidate of Chemical Sciences ‘Preparation of chiral fluorine-containing structural units and their use in the synthesis of analogues of natural compounds’ – 29.03.2002
N.O. Fokina, Dissertation for the degree of Candidate of Chemical Sciences ‘Development of methods for the synthesis of racemic and optically active fluorine-containing β-amino acids’ – 25.10.2002.
L.M. Kacharova, Dissertation for the degree of Candidate of Chemical Sciences ‘α-Halogenated β-alkoxyvinyl(polyfluoroalkyl)ketones in the synthesis of heterocyclic fluorine-containing bioregulators’ – 20.12.2002.
N.V. Liutenko, Dissertation for the degree of Candidate of Chemical Sciences ‘Synthesis of potentially bioactive compounds based on electrophilic reactions of β-aminovinyl(trifluoromethyl)ketones’ – 23.04.2004.
O.M. Shaitanova, Dissertation for the degree of Candidate of Chemical Sciences ‘Synthesis of polyfluoromethyl-containing analogues of γ-amino-butyric acid’ – 25.04.2008.
N.A. Tolmachova, Dissertation for the degree of Candidate of Chemical Sciences ‘Synthesis and chemical properties of 6-polyfluoroalkyl-containing pyrones-2’ – 25.04.2008.
I.S. Kondratov, Dissertation for the degree of Candidate of Chemical Sciences - ‘Fluorinated analogues of mevalonic and mevaldic acids’ – 15.02.2008.
K.V. Tarasenko, Dissertation for the degree of Candidate of Chemical Sciences ‘Synthesis and properties of organophosphorus compounds based on β(alkoxy,amino)vinyl(polyfluoroalkyl)ketones’ – 20.05.2011.
V.G. Dolovaniuk, Dissertation for the degree of Candidate of Chemical Sciences ‘New fluorinated analogues of ornithine, glutamic acid, proline and their derivatives’ – 05.06.2015
Feskov I.O., Dissertation for the degree of Candidate of Chemical Sciences ‘1,3-Substituted cyclobutanecarboxylic acids as structural fragments for isosteric substitutions’ – 06.05.2021
M.V. Bugera, Dissertation for the degree of Candidate of Chemical Sciences ‘The use of new difluoromethylene-containing building blocks for the synthesis of analogues to natural amino acids and nitrogen-containing heterocycles’ – 23.04.2021
I.I. Gerus, Dissertation for the degree of Doctor of Chemical Sciences ‘Synthesis of new fluorine-containing bioactive compounds based on β-alkoxyvinyl(polyfluoroalkyl)ketones’ – 28.09.2021.
A.A. Klipkov, Dissertation for the degree of Doctor of Philosophy ‘Synthesis, chemical and biological properties of 2,3-dihydro-1H-pyrrolizines and 5,6,7,8-tetrahydroindolizines based on α,β-unsaturated fluoroalkyl ketones’ – 15.02.2024.
I.G. Logvinenko, Dissertation for the degree of Candidate of Chemical Sciences ‘Synthesis of new trifluoromethoxy compounds for medicinal chemistry’ – 16.01.2025.

V.P. Kukhar Institute
of Bioorganic Chemistry and Petrochemistry
NAS of Ukraine
© 2026 IBOPC NAS of Ukraine