The history of the Laboratory dates back to the establishment of the Department of Synthesis of Physiologically Active Phosphorus Compounds in 1991. It was formed based on a Laboratory with the same name, which was part of the Department of Fine Organic Synthesis, headed by Academician of the NAS of Ukraine, Valery Pavlovych Kukhar. Since its establishment, the Department of Synthesis of Physiologically Active Phosphorus Compounds has been led by Doctor of Chemical Sciences, Professor Oleh Ivanovych Kolodiazhnyi (Corresponding Member of the NAS of Ukraine since 2012). In 2024, the Department was reorganised into Laboratory No. 1.1, Synthesis of Physiologically Active Phosphorus Compounds, within Department No. 1 of Fine Organic Synthesis, headed by Doctor of Chemical Sciences Anastasiia Olehivna Kolodiazhna.
Chemistry of phosphorus ylides. Asymmetric synthesis of organic, organoelement and organophosphorus compounds and multiple asymmetric induction. Synthesis of phosphorus analogues of natural compounds, potentially biologically active substances. Biocatalytic synthesis of optically active compounds, natural compounds, synthetic analogues, and substances with potential biological and activity.
The Laboratory research is aimed at developing preparative methods for the synthesis of organic, organoelement and organophosphorus compounds with potential biological activity, as well as at establishing principal regularities of their chemical behaviour and identifying the possibilities of using these chiral synthons in the synthesis of enantiomerically pure bioregulators.
Main scientific and practical achievements of the Laboratory
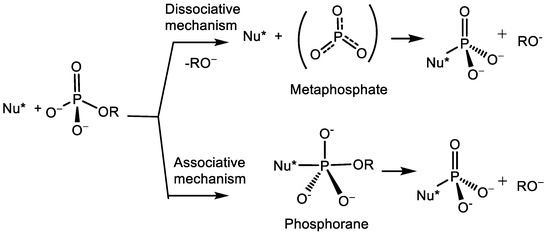
1. The stereochemistry of nucleophilic substitution near the phosphorus atom was generalised for the first time. Based on the theoretical studies performed, a preparative method for the synthesis of optically active tertiary phosphines was developed, which are important reagents in organic synthesis and are used as chiral ligands in asymmetric metal complex catalysis. The reaction mechanism was established. It was found that the SN2(P) reaction of chiral alcohols, as well as chiral amines with chlorophosphines in the presence of tertiary bases, proceeds with the transfer of chirality from the nucleophile to the phosphorus atom. The mechanism of phosphorylation-dephosphorylation of adenosine triphosphate, which is the most important energy-generating molecule that ensures the vital activity of living organisms, is proposed. The reaction proceeds by a monomolecular mechanism through the formation of a metaphosphate anion. The stereochemistry of the SN1(P) reaction was studied.
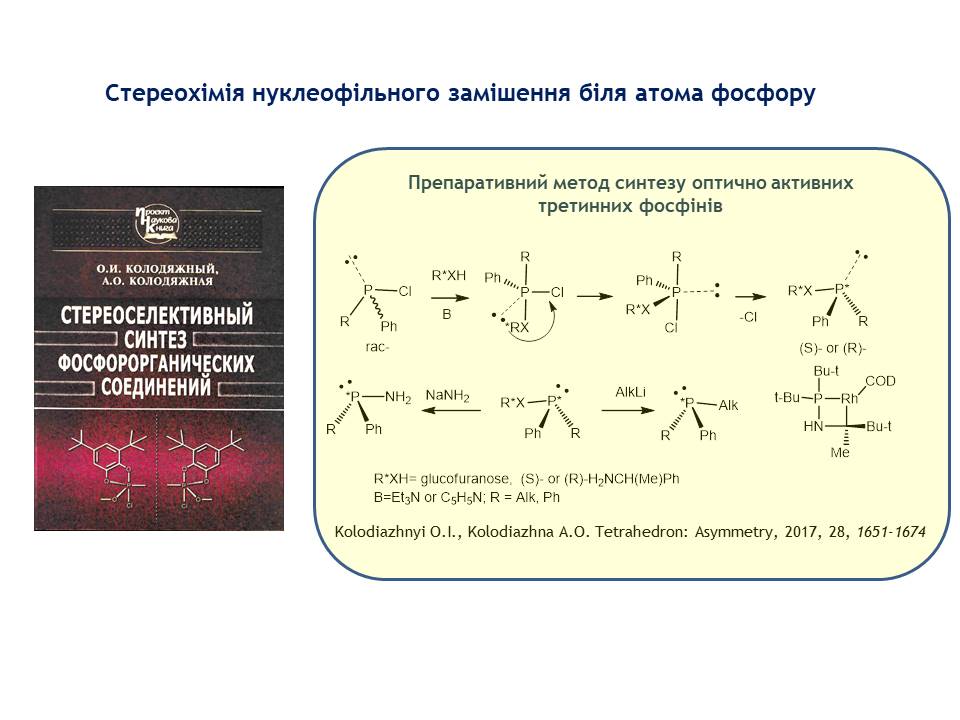
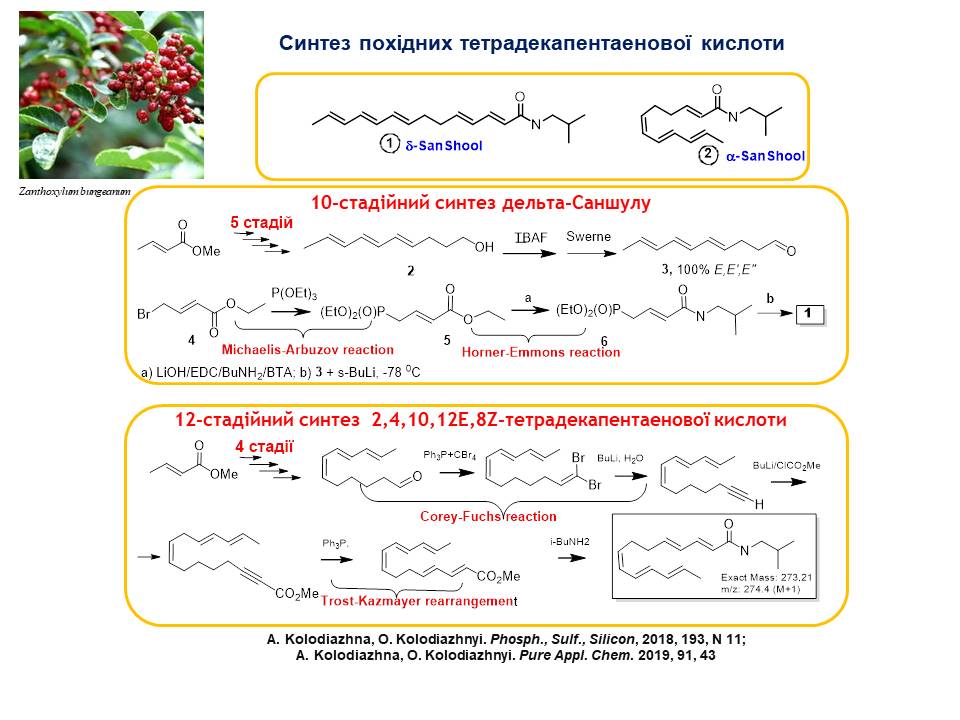
2. Stereoselective multi-step methods for the synthesis of alpha and gamma derivatives of tetradecapentanoic acid isolated from the tropical plant Zanthoxylum bungeanum (Rutaceae) have been developed, which possess interesting pharmacological properties (cell proliferation inhibitors and apoptosis activators). A 10-step method for the synthesis of trans-tetradecapentanoic acid derivatives was developed using the Corey-Fuchs reaction reaction and the Horner-Emmons reaction at key stages. In the twelve-step method for the synthesis of (2,4,10,12E, 8Z)-tetradecapentanoic acid derivatives, the Z-selective Wittig reaction, the Corey-Fuchs reaction, and the Trost-Kazmeier rearrangement were used. The synthesised substances fully correspond to natural compounds obtained from natural sources.
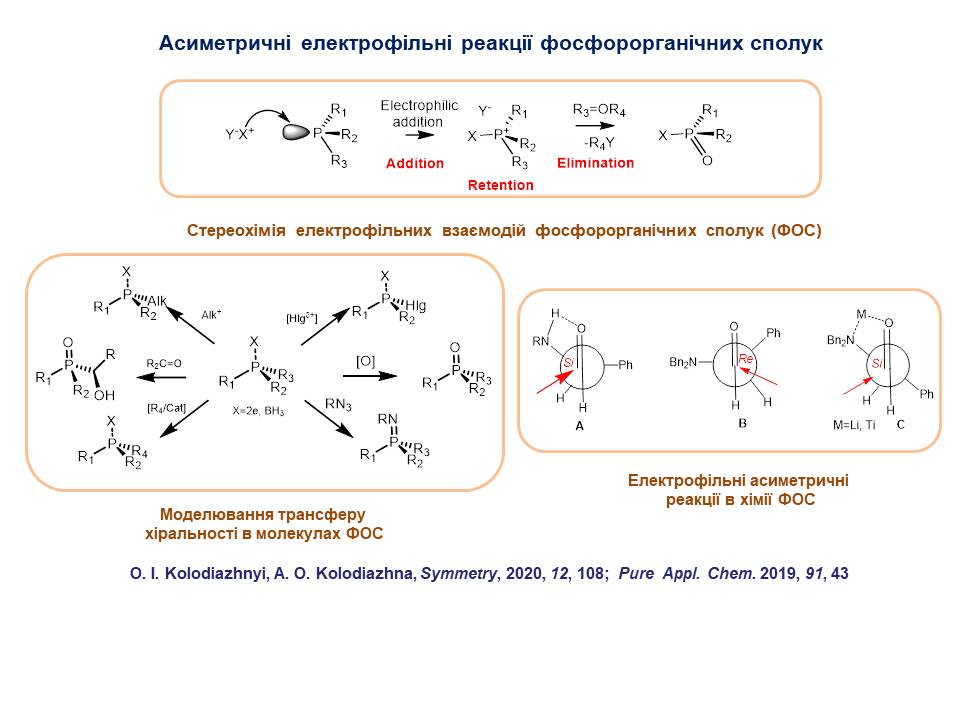
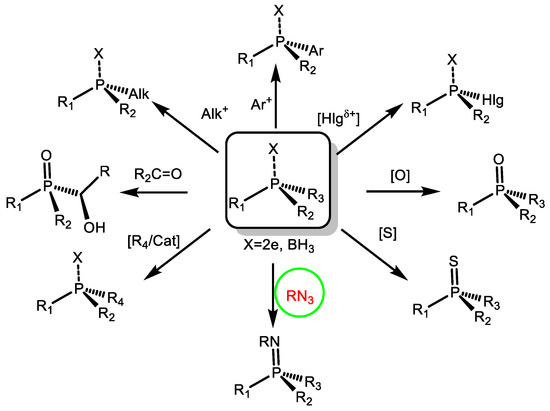
3. Electrophilic reactions of optically active organophosphorus substances were studied. A general mechanism of electrophilic interactions at the phosphorus atom was proposed. It was shown that electrophilic substitution reactions proceed stereospecifically with preservation of the absolute configuration at the phosphorus reaction centre and proceed by the addition-elimination type. The mechanism of chirality transfer from asymmetric centres to the phosphorus atom and in the reverse direction from the chiral phosphorus atom to other reaction centres was studied using the example of Hirao reactions, Staudinger ligation, halogenophilic reactions, etc. New variants of asymmetric electrophilic reactions were developed, which can be used for the synthesis of enantiomerically pure P-chiral compounds.
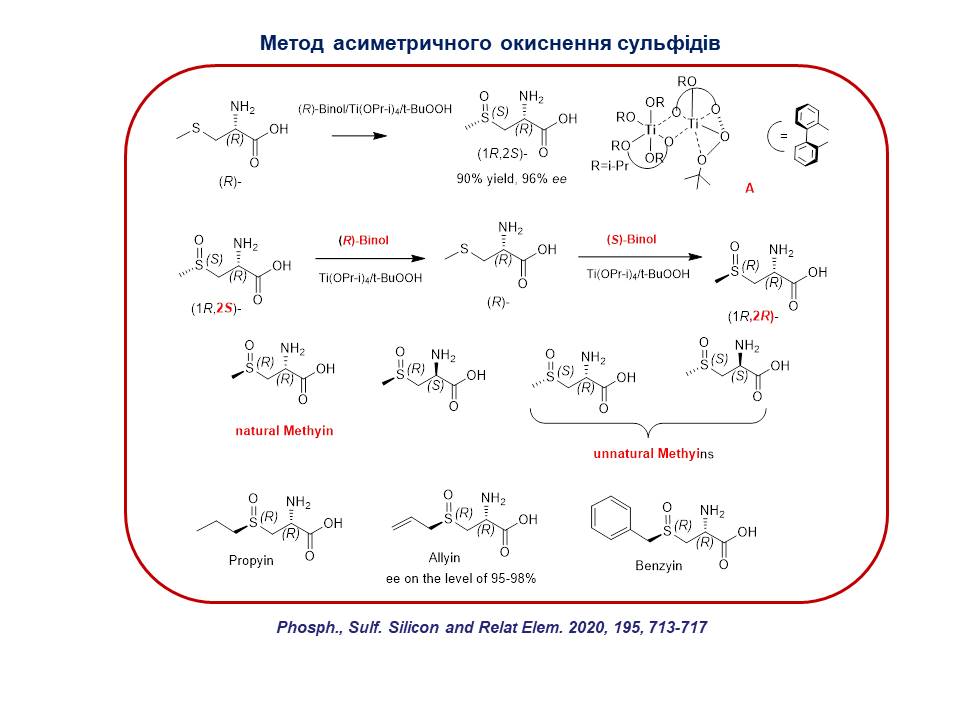
4. A general method for the asymmetric electrophilic oxidation of various asymmetric sulfides using the chiral titanium tetraisopropoxide/tert-butylhydroperoxide/(S)- or (R)-binol complex has been developed, which allows to obtain (S)- or (R)-sulfoxides with high enantiomeric excess. It has been shown that the use of a complex containing (S)-binol produces (R)-sulfoxide, and conversely, the content of (R)-binol leads to the formation of (S)-sulfoxide. This method was applied to the oxidation of sulfur-containing amino acid derivatives, which allowed to obtain a number of optically active natural sulfoxides.
5. A kinetic study of the deracemization reaction of secondary alcohols by lipases was conducted. It was established that the kinetic curve of the reaction has an S-shaped character, typical for autocatalytic reactions. Three stages of the biocatalytic esterification reaction were identified.
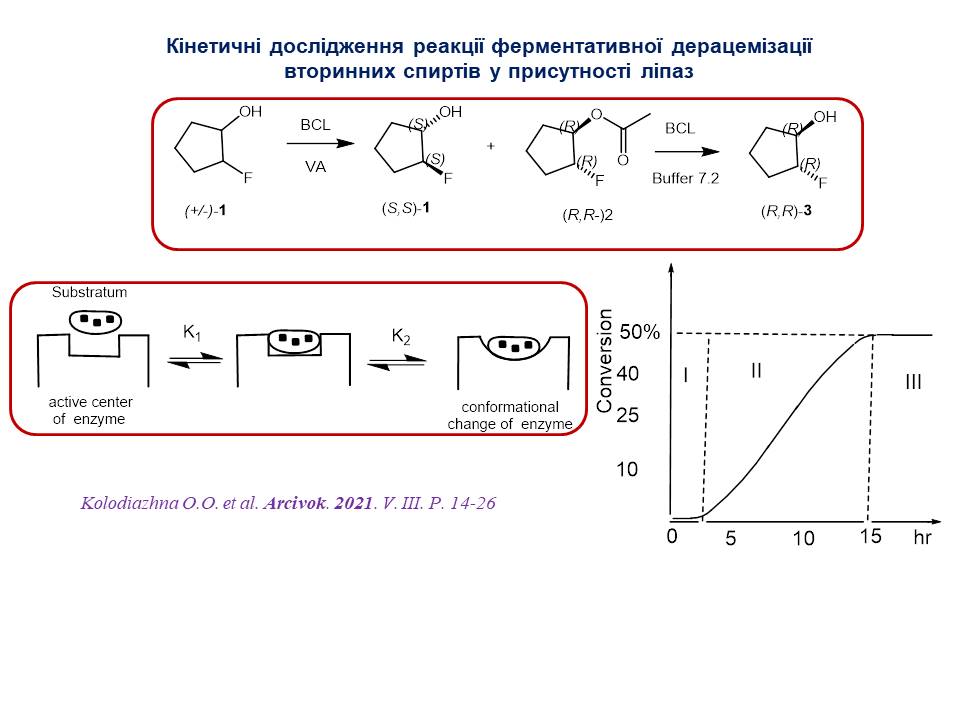
6. Methods for the synthesis of chiral phosphorus-containing analogues of natural compounds have been developed using asymmetric organocatalysis and metal complex catalysis. A highly accurate convergent method for determining the absolute configuration of five-membered cycloalkanols with the combined use of chiral HPLC, enzymatic analysis, and Kazlauskas' rule has been proposed.
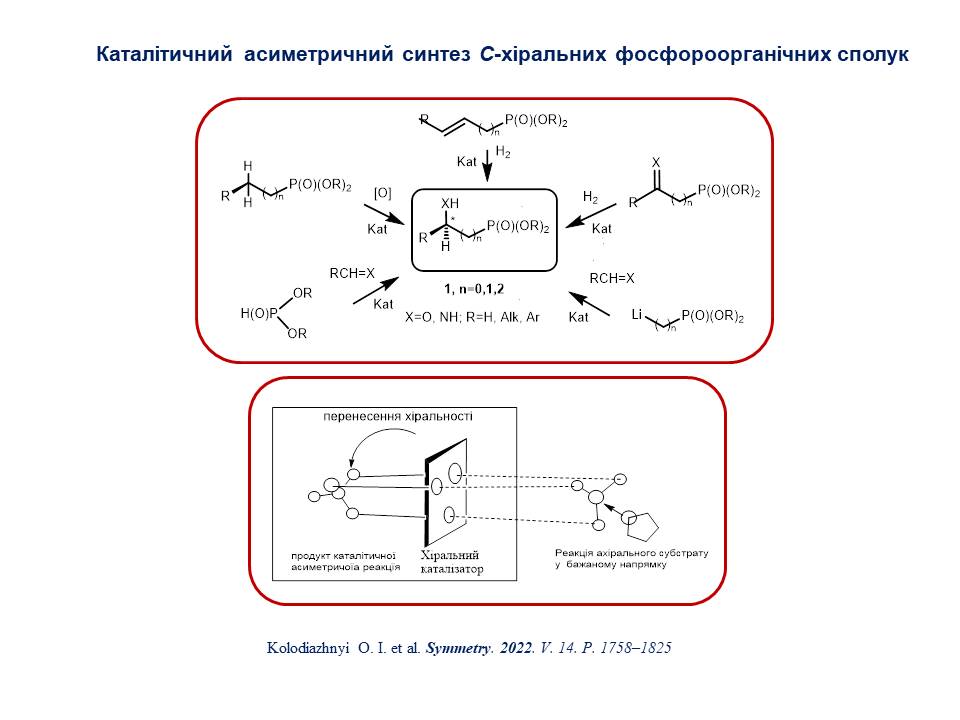
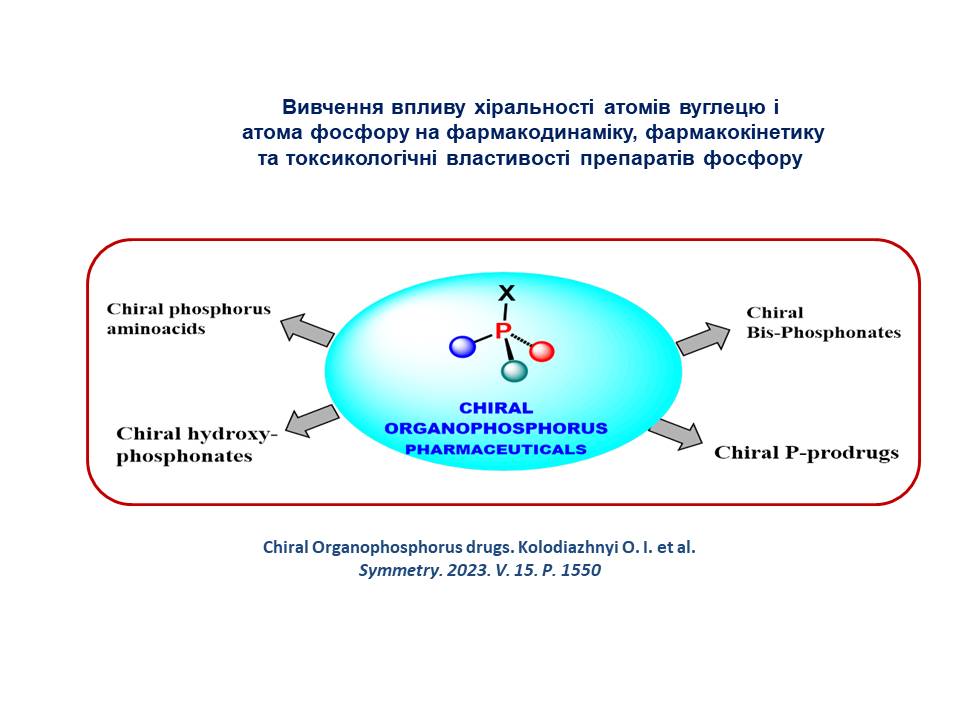
7. A new approach for the development of modern pharmaceuticals is proposed, which takes into account the regulatory norms of the US Food and Drug Administration (FDA) for chiral compounds in the development and approval of drugs. The essence of the method is the transition from racemic pharmaceuticals to enantiomerically pure representatives. Methods of virtual screening and molecular modelling allow the identification of lead compounds employing calculated free bond energies. However, due to the synthetic difficulties in obtaining enantiomerically pure stereomers and methods for their binding, it is crucial to determine the exact stereochemistry of compounds in virtual libraries of drug candidates and then consider their interaction with other biological targets. The developed set of methods that contribute to the creation of effective drugs was called "Chiral technologies", among which the technologies, namely "Chiral switches" and "Chiral Prodrugs", are of greatest interest.
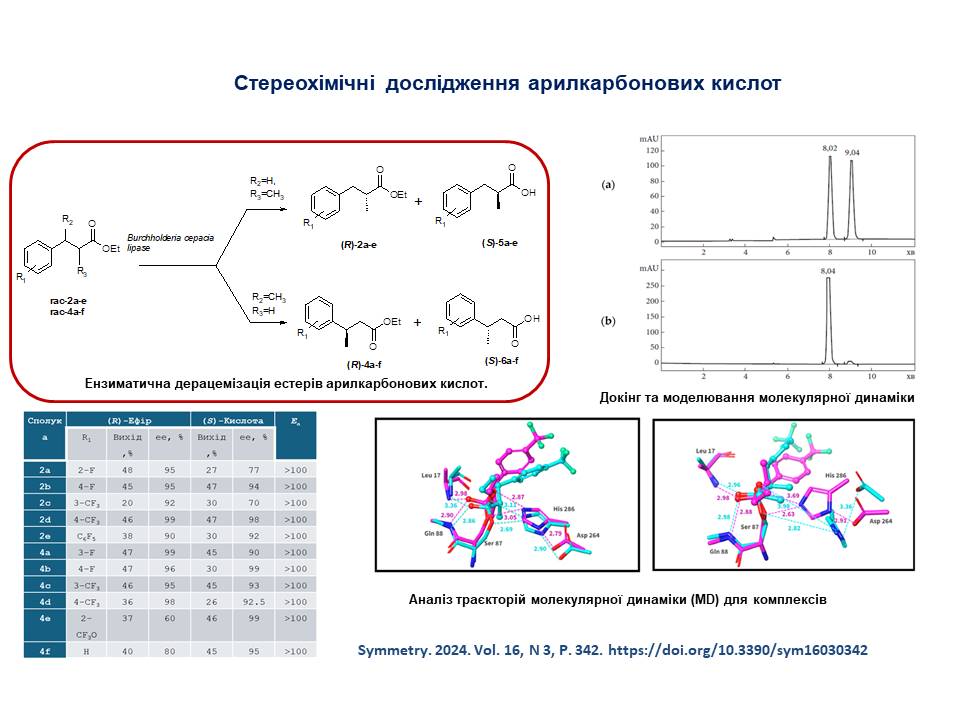
8. Methods for the synthesis of enantiomerically pure 3-aryl-2-methylpropanoates and 3-arylbutanoates using the enzymatic deracemization methodology have been developed.
Scientists of the Laboratory

1 Category Engineer, Candidate of Chemical Sciences, Evgen Grysgkun
https://www.scopus.com/authid/detail.uri?authorId=6603458102&origin=resultslist

Research Fellow, Candidate of Chemical Sciences, Serhiy Sheiko

Junior Research Fellow, PhD, Dmytro Prysyazhnyuk
https://orcid.org/0000-0003-3286-4316

1 Category Engineer, Candidate of Chemical Sciences, Olha Kolodiazhna
https://orcid.org/0000-0002-2438-5895
https://www.scopus.com/authid/detail.uri?authorId=26537545400&origin=resultslist

PhD Student, Oleh Faiziev
The most important publications of the Laboratory researchers
1. Колодяжный О.И. Химия илидов фосфора Киев: “Наукова думка”, 1994. C.1-560. (монографія).
2. Kolodiazhnyi O.I. New achievements in asymmetric synthesis of organophosphorus compounds.advances of asymmetric synthesis (Eds Alfred Hassner). JAI Press Inc. Stamford-London 1998. Vol. 3, P. 273-323. (chapter in monograph).
3. Kolodiazhnyi O.I. Phosphorus Ylides. Chemistry and Application in Organic Synthesis J. Wiley-VCH. Weinheim-New York-Chichester. 1999, P.1-565. (monograph).
4. О. И. Колодяжный, А. О. Колодяжна. Стереоселективный синтез фосфорорганических соединений. «Наукова Думка», 2017, 342 с. (монографія)
5. Kolodiazhnyi O. I. Multiple stereoselectivity and its application in organic synthesis. Tetrahedron, 2003, Vol. 59, P. 5953-6018. (review) https://doi.org/10.1016/S0040-4020(03)00911-6

6. Kolodiazhnyi O. I. Asymmetric synthesis of hydroxyphosphonates. Tetrahedron: Asymmetry, 2005, Vol. 16, P. 3295-3340. (review). https://doi.org/10.1016/j.tetasy.2005.09.007

7. Guliaiko, Irina; Nesterov, Vitaly; Sheiko, Sergei; Kolodiazhnyi, Oleg I.; Freytag, Matthias; Jones, Peter G.; Schmutzler, Reinhard. Synthesis of optically active hydroxyphosphonates. Heteroatom Chemistry, 2008, vol. 19, # 2, p. 133 - 139. https://onlinelibrary.wiley.com/doi/10.1002/hc.20391
8. Kachkovskyi, Georgiy O.; Kolodiazhnyi, Oleg I. Synthesis of phosphonic acids possessing isoindolin-1-one moiety: Unexpected acid-catalyzed С-P-bond cleavage. Phosphorus, Sulfur and Silicon and the Related Elements, 2009, vol. 184, # 4, p. 890 – 907. https://doi.org/10.1080/10426500802715601
9. Kolodiazhnyi, Oleg I.; Gryshkun, Evgen V.; Kolodiazhna, Anastasya O.; Kachkovskyi, Georgy O.; Kolodiazhna, Olga O.; Sheiko, Sergyi Yu; Zemlianoi, Viacheslav N. New methods for the synthesis of phosphonic analogues of natural compounds. Phosphorus, Sulfur and Silicon and the Related Elements, 2011, vol. 186, # 4, p. 644 – 651. https://doi.org/10.1080/10426507.2010.511359
10. Gryshkun, Yevgen V.; Nesterov, Vitalyi M.; Kolodyazhnyi Oleh I. Enantioselective reduction of ketophosphonates using adducts of chiral natural acids with sodium borohydride. Arkivoc, 2012, vol. 2012, # 4, p. 100 – 117. https://doi.org/10.3998/ark.5550190.0013.409
11. Kolodiazhnyi, Oleg I. Recent developments in the asymmetric synthesis of -chiral phosphorus compounds. Tetrahedron: Asymmetry, 2012, vol. 23, # 1, p. 1 – 46. (review) https://doi.org/10.1016/j.tetasy.2012.01.007

12. Kolodiazhnyi, Oleg I. Advances in asymmetric hydrogenation and hydride reduction of organophosphorus compounds. Phosphorus, Sulfur and Silicon and the Related Elements, 2014, vol. 189, p. 1102 – 1131. (review) https://doi.org/10.1080/10426507.2014.905778

13. Kolodiazhnyi, Oleg I.; Kukhar, Valery P.; Kolodiazhna, Anastasy O. Asymmetric catalysis as a method for the synthesis of chiral organophosphorus compounds. Tetrahedron Asymmetry, 2014, vol. 25, # 12, p. 865 - 922. (review) https://doi.org/10.1016/j.tetasy.2014.05.010

14. Kolodiazhna, Anastasia O.; Kolodiazhnyi, Oleg I. Synthesis and properties of four-membered phosphorus heterocycles-2-Fluoro-1,2λ5-Oxaphosphetanes. Phosphorus, Sulfur and Silicon and the Related Elements, 2015, vol. 190, # 12, p. 2232 – 2245. https://doi.org/10.1080/10426507.2015.1054485

15. Kolodiazhnyi, Oleg I.; Kolodiazhna, Anastasy O.Multiple stereoselectivity in organophosphorus chemistry. Phosphorus, Sulfur and Silicon and the Related Elements, 2016, vol. 191, # 3, p. 444 – 458. (review) https://doi.org/10.1080/10426507.2015.1091831

16. Kolodiazhnyi, Oleg I.; Kolodiazhna, Anastasy. Nucleophilic substitution at phosphorus: stereochemistry and mechanisms. Tetrahedron Asymmetry, 2017, vol. 28, # 12, p. 1651 – 1674. https://doi.org/10.1016/j.tetasy.2017.10.022

17. Kolodiazhnyi, Oleg I.; Kolodiazhna, Anastasy O. Stereochemistry of nucleophilic substitution at trivalent phosphorus. Phosphorus, Sulfur and Silicon and the Related Elements, 2017, vol. 192, # 6, p. 621 – 633. https://doi.org/10.1080/10426507.2017.1284842

18. Kolodiazhnyi, Oleg I. Stereochemistry of electrophilic and nucleophilic substitution at phosphorus. Phosphorus, Sulfur and Silicon and the Related Elements, 2019, vol. 194, # 4-6, p. 396 – 400. https://doi.org/10.1080/10426507.2018.1521409

19. Kolodiazhnyi, Oleg I. Stereochemistry of electrophilic and nucleophilic substitutions at phosphorus. Pure and Applied Chemistry, 2019, vol. 91, # 1, p. 43 – 57. https://doi.org/10.1515/pac-2018-0807

20. Kolodiazhna, Anastasy; Kolodiazhnyi, Oleg. Stereoselective syntheses of sanshool derivatives. Phosphorus, Sulfur and Silicon and the Related Elements, 2019, vol. 194, # 4-6, p. 275 – 276. https://doi.org/10.1080/10426507.2018.1514404

21. Kolodiazhna, Anastasy O.; Kolodiazhnyi, Oleg I. Asymmetric electrophilic reactions in phosphorus chemistry. Symmetry, 2020, vol. 12, # 1, art. no. 108. https://doi.org/10.3390/sym12010108
22. Kolodiazhna, Olga O.; Prysiazhnuk, Dmitry V.; Kolodiazhna, Anastasy O.; Kolodiazhnyi, Oleg I. Synthesis of optically active vicinal fluorocyclopentanols and fluorocyclopentanamines by enzymatic deracemization. Arkivoc, 2021, vol. 2022, # 3. https://doi.org/10.24820/ark.5550190.p011.634
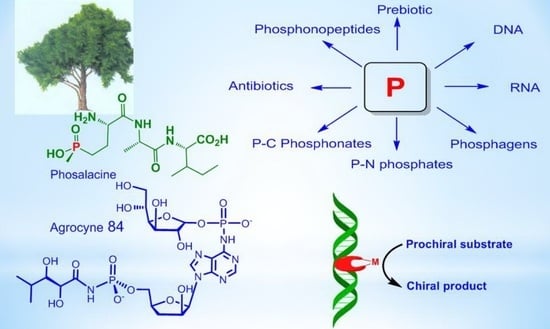
23. Kolodiazhnyi, Oleg I. Phosphorus compounds of natural origin: Prebiotic, stereochemistry, application. Symmetry, 2021, vol. 13, # 5, art. no. 889. https://doi.org/10.3390/sym13050889
24. Kolodiazhna, Anastasy O.; Kolodiazhnyi, Oleg I. Catalytic Asymmetric Synthesis of C-Chiral Phosphonates. Symmetry, 2022, vol. 14, # 9, art. no. 1758. https://doi.org/10.3390/sym14091758

25. Kolodiazhnyi, Оleg; Kolodiazhna, Anastasy; Grishkun, Evgen; Prysiazhnuk, Dmitry; Kolodiazhna, Olga; Sheiko, Sergei. Achievements in developments of organophosphorus stereochemistry. Phosphorus, Sulfur and Silicon and the Related Elements, 2022, vol. 197, # 5-6, p. 474 – 479.https://doi.org/10.1080/10426507.2021.2011874

26. Kolodiazhna, Anastasy O.; Kolodiazhnyi, Oleg I. Chiral Organophosphorus Pharmaceuticals: Properties and Application. Symmetry, 2023, vol. 15, # 8, art. no. 1550. https://doi.org/10.3390/sym15081550
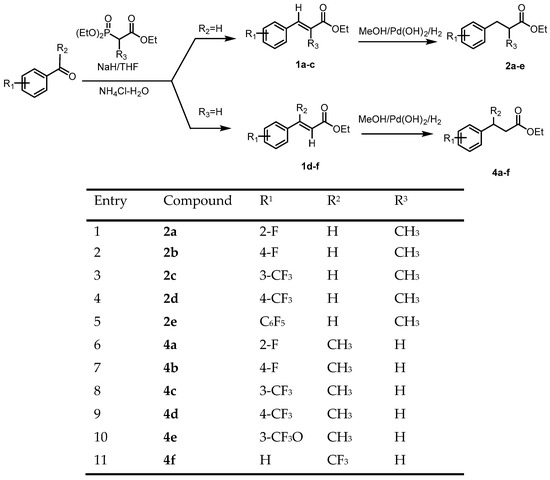
27. Kolodiazhnyi, Oleg I.; Kolodiazhna, Anastasiia O.; Faiziiev, Oleh; Gurova, Yuliia. Enzymatic Deracemization of Fluorinated Arylcarboxylic Acids: Chiral Enzymatic Analysis and Absolute Stereochemistry Using Chiral HPLC. Symmetry, 2024, vol. 16, # 9, art. no. 1150. https://doi.org/10.3390/sym16091150
28. Kolodiazhna, Anastasy; Prysiazhnuk, Dmitry; Kolodiazhnyi, Oleg. Enzymatic resolution of heterocyclic intermediates for biologically active compound preparation. Phosphorus, Sulfur and Silicon and the Related Elements, 2024, vol. 199, # 10-12, p. 843 – 855. https://doi.org/10.1080/10426507.2024.2367033

29. Kolodiazhnyi, Oleg I.; Kolodiazhna, Anastasy O. Stereoselective Syntheses of Organophosphorus Compounds. Symmetry, 2024, vol. 16, # 3, art. no. 342. https://doi.org/10.3390/sym16030342

Laboratory staff (March 2025). From left to right: Faiziev O.O., Kolodyazhna O.O., Prysyazhnyuk D.V., Kolodyazhna A.O., Sheiko S.Yu., Grishkun E.V.

Corresponding Member of the NAS of Ukraine Kolodyazhny O.I. with his Polish colleagues at the XX International Symposium "Achievements in the Chemistry of Heteroorganic Compounds", 2017, Lodz, Poland.

Kolodyazhna A.O. at the 18th European Symposium on the Chemistry of Organofluorine Compounds, 2016, Kyiv, Ukraine.

Захист докторської дисертації Колодяжної А.О. 2017 р.

Defense of the doctoral dissertation of Kolodyazhna A.O. 2017.
Report of graduate student Prysyazhnyuk D.V. at the XXVIII Scientific Conference on Bioorganic Chemistry and Petrochemistry, 2022.
V.P. Kukhar Institute
of Bioorganic Chemistry and Petrochemistry
NAS of Ukraine
© 2026 IBOPC NAS of Ukraine