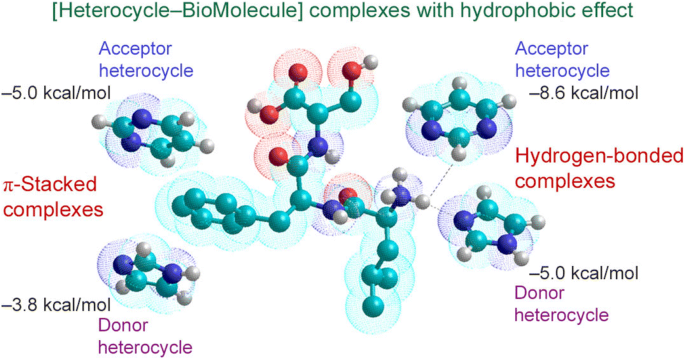
|
Diphosphorus P2: A New Synthetic Tool in Organophosphorus Chemistry.
|
Current Organic Chemistry. 2025. V. 29 |
Romanenko V.D. |
|
In silico modeling and experimental validation of 2-oxoimidazolidin-4-sulfonamides as low-toxicity fungicides against Phytophthora infestans.
|
Advanced Agrochemicals 2025. V. 4, N 2. P. 141-148. |
V. Kovalishyn, D. Hodyna, I. Semenyuta, V. Brovarets, O. Shablykin, S. Chumachenko, L. Metelytsia |
|
In Silico and In Vitro Studies of 1,3-Oxazol-4-yl Phosphonium Salts as Dual-Functional Antibacterial and Anticancer Agents.
|
ChemistrySelect. 2025. V. 10, N 20. e00282 |
D. Hodyna, O. Bahrieieva, Y. Shulga, V. Kovalishyn, O. Golovchenko, M. Kachaeva, S. Pilyo, O. Trokhimenko, L. Metelytsia, V. Brovarets |
|
Acute Toxicity of Quinoline Derivatives as Antibacterial Drug Candidates: In Silico and In Vivo Studies.
|
Bioactivities. 2025. V. 3, N 1. P. 25-39. |
D. Hodyna, V. Kovalishyn, O. Brazhko, L. Metelytsia |
|
Ionic liquids and lysosomotropic detergents as inhibitors of the SARS-CoV-2 main protease: QSAR modeling, synthesis and biological testing.
|
Biochemical and Biophysical Research Communications. 2025. V. 777. 152276 |
D. Hodyna, A. Gryniukova, S. Rogalsky, P. Borysko, V. Kovalishyn, L. Metelytsia |
|
Enhancement of sensory properties of chemoresistive conductive nanocomposites of carbon nanotubes and polypyrrole by functionalized calixarenes. |
Semiconductor Physics, Quantum Electronics & Optoelectronics. 2025. V. 28, № 1. P. 109-120 |
A.A. Pud, Yu.V. Noskov, N.A. Ogurtsov, O.L. Kukla, O.S. Kruglyak, A.V. Mamykin, S.O. Cherenok, S.G. Vyshnevskyy, V.I. Kalchenko |
|
Design, synthesis and antitumor potential of new thiazole-contained 5-fluoro-2-ox-indole as Sunitinib analogues. |
Current Medicinal Chemistry. 2025. V. 32, N 25. P. 5279-5291. |
Semenyuta I., Los. O., Sinenko V., Zhirnov V., Potikha L., Kobzar O., Brovarets V. |
|
Design, synthesis and anticancer activity of novel 4-(5-amino-4-cyano-1,3-oxazol-2-yl)benzenesulfonamide derivatives. |
Current Chemistry Letters. 2025. N 14. P. 159-172. https://doi.org/10.5267/j.ccl.2024.8.001 |
Severin O., Piyo S., Semenyuta I., Kachaeva M., Zhirnov V., Brovarets V. |
|
Synthesis of enantioenriched 2-((hetera)cyclo)alkylchromanols and their spirocyclic analogs through enzymatic resolution.
|
European Journal of Organic Chemistry. 2025. V. 28, N8. e202401247. https://doi.org/10.1002/ejoc.202401247
|
Timokhin O.S., Romanova A.M., Moskvina V.S., Kucher O.V., Boiko A.I., Banasevych A.L., Darylin D., Brovarets V.S., Grygorenko O.O. |
|
[3+2]Cycloaddictions of α,β-unsaturated sultams. |
ChemRxiv. 2025. V. 28, N 18. E202500093. https://doi.org/10.26434/chemrxiv-2024-bg6fk
|
Zaika Y.O., Vashchenko B.V., Sosunovych B.S., Khutorianskyi A.V., Yasman P., Ogurok V.M., Brovarets V.S., Grygorenko O.O. |
|
Desighn, synthesis, SHR and ADMET analyses of the novel class of synthetic 7-amino[1,2,5]oxadiazolo[3,4-b]pyridine-6-carboxylate derivatives with vasorelaxant activity.
|
Results in Chemistry. 2025. V. 13. e102031. https://doi.org/10.1016/j.rechem.2025.102031 |
Varenyk A., Vydzhak R., Panchishin S., Ivanova I., Zhirnov V., Brovarets V. |
|
Bridged bicyclic ɣ-sultames by intramolecular Flow photochemical [2+2]cycloaddition.
|
Organic Letters. 2025. V. 27, N 12. P. 2858-2862. https://doi.org/10.1021/acs.orglett.5c00314 |
Zaika Ye., Borodin I., Olekh H., Kovalov M., Diachenko O., Brovarets V., Vashchenko B., Grygorenko O. |
|
An atlas of metabolites driving chemotaxis in prokaryotes. |
Nature Communications. 2025. V. 16, N 1. |
Brunet M., Amin S.A., Bodachivskyi Yu., Kuzhivmparonibil U., Seymour J.R., Paina J.-B. |
|
Mechanochemical synthesis of 5-amino-4-cyanoxazoles.
|
European Journal of Organic Chemistry. 2025. V. 28, N 21. e202500145. https://doi.org/10.1002/ejoc.202500145 |
Merzhyievskyi D., Shablykin O.V., Jarg T., Brovarets V.S., Aav R., Kananovich D.G. |
|
New 4-benzenesulfonamide derivatives of pyrazolo[1,5-a][1,3,5]triazine as purine bioisosteres: development, synthesis, and anticancer perspective. |
Current Medicinal Chemistry. 2025. https://doi.org/10.2174/0109298673385989250711112625
|
Semenyuta I., Pilyo S., Demydchuk B., Lyavinets O., Brovarets V., Velihina Ye. |
|
Impact of terminal group on temperature-dependent excited state relaxation in cationic dyes.
|
Chemical Physics. 2025. V. 592. |
Piryatinski Y., Verbitsky A., Malynovskyi M., Rozhin A., Kachkovsky O., Maiko K., Slominskii Yu., Lutsyk P. |
|
Effect of nitrogen atom position in Sudan 1-like azo-naphtalene dyes on linear and nonlinear optical properties: DFT and experimental study.
|
Journal of Molecular Structure. 2025. V. 1333. P. 141735. |
Ovdenko V.M., Labunets A.R., Volochniuk M.O., Ronkovych A.V., Komarenko D.O., Kachkovsky O.D., Gayvoronsky V.Ya. |
|
Design, synthesis, anticancer screening and virtual analysis of new 7-sulfonyldiazepan- and 7-sulfonylpiperazine substituted oxazolo[4,5-d]pyrimidines.
|
SynOpen. 2025. V. 9. P.186-202.
|
Pilyo S., Kachaeva M., Zhirnov V., Kovalishyn V., Velihina Ye., Semenyuta I., Metelytsia L., Brovarets V. |
|
N-(5-(Dichloromethylene)-2-oxoimidazolidin-4-ylidene)sulfonamides: Small molecules with big synthetic capabilities. |
Current Chemistry Letters. 2025. V.14. P. 817-830. |
Chumachenko S.A., Shablykin O.V., Shishkina S.V., Kozytskyi A.V., Shablykina O.V. |
|
Influence of the plant growth regulator Kamethur on the morphological features and yield of Chinese cowpea (Vigna sinensis L.). |
Sciences of Europe. 2025. V. 166, N 166. P. 3-17. DOI: 10.5281/zenodo.15654466. |
Kovalenko O.A., Mikolaychuk V.G., Tsygankova V. A., Andreev A.M., Pilyo S.G., Brovarets V.S. |
|
Application of New Maize Growth Regulators Based on Furopyrimidine Derivatives under Heat and Drought Stress Conditions. |
Nutri Food Sci Int J. 2025. V. 14, N 4. Р. 555893. DOI: 10.19080/NFSIJ.2025.14.555893 |
Tsygankova V.A., Andrusevich Ya.V., Vasylenko N.M., Kopich V.M., Solomyannyi R.M., Pilyo S.G., Kachaeva M.V., Bondarenko O.M., Kozachenko O.P., Popilnichenko S.V., Brovarets V.S. |
|
Novel Pyrimidine Derivatives as Regulators of Barley Growth and Photosynthesis During the Vegetation Period. |
J. Nutrition and Food Processing. 2025. V. 8 N 7. Р. 1-12. |
Tsygankova V.A., Vasylenko N.M., Kopich V.M., Solomyannyi R.M., Kachaeva M.V., Pilyo S.G., Kozachenko O.P., Bondarenko O.M., Popilnichenko S.V., Brovarets V.S. |
|
Helicobacter pylori CagA protein is a potent and broad-spectrum amyloid inhibitor. |
Science Advances. 2025. V. 11, N 24. eads7525. http://doi.org/10.1126/sciadv.ads7525 |
Jin Z., Olsen W.P., Mörman C., Leppert A., Kumar R., Møllebjerg A., Nielsen L.G., Moshynets O. V., Frasinyuk M.S., Elosua J.Y., Ferreira D., Abelein A., Landreh M., Knight S. D., Johansson J., Otzen D. E., Chen G. |
|
A novel amino-pyrimidine inhibitor suppresses tumor growth via microtubule destabilization and Bmi-1 down-regulation.
|
Biochemical Pharmacology. 2025. V. 233. P. 116783. https://doi.org/10.1016/j.bcp.2025.116783 |
Gao L., Liu J., Zhang R., Chen X., Wang M., Dong Y., Frasinyuk M.S., Zhang W., Watt D., Meng W., Xue J., Liu C., Cheng Y., Liu X. |
|
Divergent synthesis of novel 3(5)-aminoazole–benzopyrone hybrids and their evaluation as α-glucosidase inhibitors.
|
ChemMedChem. 2025. V. 20. e202400525. https://doi.org/10.1002/cmdc.202400525 |
Myshko A.S., Mrug G.P., Bondarenko S.P., Demydchuk B.A., Kobzar O.L., Buldenko V.M., Vovk A.I., Frasinyuk M.S. |
|
P(O)Me2-Alkenes: from synthesis to applications.
|
Organic Letters. 2025. V. 27, N 23. P. 6174-6178. https://doi.org/10.1021/acs.orglett.5c01805 |
Melnychuk P.V., Kulish O.V., Pavlish S.I., Kubyshkin V., Mykhailiuk P.K. |
|
Red Cabbage Anthocyanin-Loaded Bacterial Cellulose Hydrogel for Colorimetric Detection of Microbial Contamination and Skin Healing Applications.
|
Polymers. 2025. V. 17, N 15. Р. 2116 |
Melnyk H., Havryliuk O., Zaets I., Sergeyeva T., Zubova G., Korovina V., Scherbyna M., Savinska L., Khirunenko L., Amler E., Bardosova M., Gorbach O., Rogalsky S., Kozyrovska N. |
|
Impact of N-hexanoyl-DL-homoserine lactone Priming on Hormonal Homeostasis in Winter Wheat.
|
Plant Physiology and Biochemistry. 2025. V. 229, Part E. Р. 110809 |
Babenko L., Voytenko L., Futorna O., Shcherbatiuk M., Vasyuk V., Romanenko K., Rogalsky S., Kosakivska I. |
|
Epoxy resin/liquid composite as promising coating material with improved toughness and antibiofilm activity.
|
Coatings. 2025. V. 15, N 7. Р. 821 |
Rogalsky S., Moshynets O., Dzhuzha O., Lobko Y., Hubina A., Darabut A.M., Romanenko Y., Tarasyuk O., Potters G. |
|
Synthesis and acetylcholinesterase inhibitory activity of novel trilaciclib analogs.
|
Chemistry & Biodiversity. 2025. V. 22, N 2. e202401874. https://doi.org/10.1002/cbdv.202401874
|
L. Muzychka, O. Muzychka, O. Smolii |
|
Synthesis and structure of thiacalix [4] arene phosphoric acids and their ability to inhibit protein tyrosine phosphatases.
|
Phosphorus, Sulfur, and Silicon and the Related Elements. 2025. Р. 1-12. |
Silenko O., Cherenok S., Kobzar O., Shulha Y., Rusanov E., Karpichev Y., Drapailo A., Vovk A., Kalchenko V.
|
|
Synthesis, anticancer screening, and virtual analysis of 5-S-substituted derivatives of 1, 3-oxazol-4-ylphosphonates and 1, 3-oxazol-4-carbonitriles.
|
SynOpen. 2025. V. 9, N 04. Р. 268-281 DOI: 10.1055/a-2741-9575 |
Bahrieieva O.S., Kachaeva M.V., Kobzar O.L., Shulga Y.V., Golovchenko O.V., Golovchenko O.I., Nizhenkovska I.V., Mykhailenko O.V., Pilyo S.G., Zhirnov V.V., Brovarets V.S. |
|
The role of zinc in regulation of plant metabolism: what is known to date? |
J. Plant Growth Regul. 2025. https://doi.org/10.1007/s00344-025-11975-2 |
Y. Kolesnikov, S. Kretynin, V. Markhaichuk, R. Filepova, P.I. Dobrev, J. Martinec, O. Shablykin, T. Schmülling, V. Kravets |
|
Brassinosteroid Synthesis and Perception Differently Regulate Phytohormone Networks in Arabidopsis thaliana. |
Int. J. Mol. Sci. 2025. V. 26, N 19. Р. 9644 |
Bukhonska Y., Derevyanchuk M., Filepova R., Martinec J. Dobrev P., Ruelland E., Kravets, V. |
|
Synthesis of Optically Active Indane Building Blocks Using Enzymatic Resolution Approach.
|
Tetrahedron. 2025. V. 184. 134759. |
Banasevych A., Kucher O., Sosunovych B., Nazarenko N., Shevchenko D., Smolii O., Vashchenko B. |
|
Synthesis, structure, and antiviral activity 4(6)-β-d-glucopyranosylamino-2-R-1,3-benzothiazoles.
|
Carbohydrate Research. 2025. V. 558. 109700 |
Guzyr O.I., Potikha L.M., Shishkina S.V., Fetyukhin V.N., Shermolovich Y.G., Bas J.P., Kulyk I.B., Zaremba P.Y., Zahorodnia S.D. |
|
Fluorine in the Pharmaceutical Industry: FDA‐Approved Fluorine‐Containing Drugs in 2024.
|
Chem. Eur. J. 2025. V. 31, N 25. e202500662 |
Du Y., Bian Y., Baecker D., Dhawan G., Semghouli A., Kiss L., Zhang W., Sorochinsky A.E., Soloshonok V.A., Han J. |
|
An engineered cereblon optimized for high-throughput screening and molecular glue discovery.
|
Cell Chemical Biology. 2025. V.32. Р. 363-376 |
Bailey H.J., Eisert J., Kazi R., Gerhartz J., Pienkowska D.E., Dressel I., Vollrath J., Kondratov I., Matviyuk T., Tolmachova N., Shah V.J., Giuliani G., Mosler T., Geiger T.M., Esteves A.M., Santos S.P., Sousa R.L., Bandeiras T.M., Leibrock E.-M., Bauer U., Leuthner B., Langer J.D., Wegener A.A., Nowak R.P., Sorrell F.J., Dikic I. |
|
Synthesis and Physicochemical Characterization of 6-Trifluoromethyl Spiro[3.3]Heptane Building Blocks.
|
European Journal of Organic Chemistry. 2025. V.28. e202500587 |
Olifir O.S., Lenda P.R., Chernykh A.V., Klymenko D.S., Liashuk O.S., Shishkina S.V., Kondratov I.S., Grygorenko O.O. |
|
Synthesis and Physicochemical Characterization of 5 Trifluoromethyl-Substituted Saturated O- and S-Heterocycle
|
European Journal of Organic Chemistry 2025. V.28. e202501086 https://doi.org/10.1002/ejoc.202501086
|
Redka M.O., Dovhaniuk N., Blahun O., Liashuk O.S., Hurieva A.M., Lesyk D., Skrypnik D., Holota Y., Durylin D., Borysko P., Kondratov I.S., Grygorenko O.O. |
|
Saturated F2-Rings from Alkenes.
|
Angewandte Chemie. 2025. V.137, N 914. e202422899 |
Li Y., Liu X.-B., Sham V., Logvinenko I., Xue J.-H., Wu J.-Y., Fu J.-L., Lin S., Liu Y., Li Q., Mykhailiuk P.K., Wang H. |
|
Structure-Activity Relationship Studies Towards Analogues of Pleconaril as Novel Enterovirus-D68 Capsid-Targeting Antivirals. |
ВioRxiv preprint doi:
|
Cousins D.L., Griffen E.J., Stacey J., Lee A.A., Filimonova Y., Hlavin A., Holota Y., Khmil R., Kordubailo M., Kostinov O., Lesyk D., Logvinenko I., Lototska M., Lysenko V., Pashchenko A., Pavlichenko M., Rodnichenko A., Tkachenko A., Hurst B.L., Julander J.G., Wang H., Pearl R., Benjamin J., Diaz-Tapia R., Gordon M.E., Albrecht R.A., White K. |
|
Development and turbine engine testing of coconut oil-based biojet fuel.
|
Fuel Proc. Technol. 2025. V. 274, Р. 108252. https://doi.org/10.1016/j.fuproc.2025.108252 |
Yakovlieva A., Andoga R., Főző L., Zubenko S., Konovalov S. |
|
Properties of components of renewable motor fuel based on plant oils and assessment of their compatibility with traditional fuels. |
Energies. 2024. V.17, N 24. Р. 6390 |
Boichenko S., Yakovlieva A., Zubenko S., Konovalov S., Shkilniuk І., Artyukhov A., Wit B., Czarnocki K. |
|
Ni-Bi-Mo/Al2O3 Metal-Oxide Catalysts for CO2-Mediated Oxidative Dehydrogenation
|
Catalysis Letters. 2025. V. 155, N 8. Р. 266. |
O.V. Larina, O.V. Zikrata, O.P. Pertko, I.M. Remezovskyi, P.I. Kyriienko, S.O. Soloviev |
|
Physical, Chemical, and Performance Properties of Biodiesel Fuels: A Comparative Study of Lipid-Based Feedstocks. |
Energies. 2025. V. 18, N 16. Р. 4274. |
Boichenko S., Yakovlieva A., Zubenko S., Shkilniuk І. |
|
Degradation of Congo Red dye on zeolite. |
Research Square, Preprints. 2025. |
Patrylak L, Yakovenko A., Nizhnik B. |
|
Surfactant-modified Transcarpathian natural zeolite as a sorbent of organic dyes from aqueous solutions. |
ChemRxiv. Preprints. 2025. 11 p. https://chemrxiv.org/engage/chemrxiv/article-details/6902508cef936fb4a20d7685 |
Voloshyna Yu., Pertko O., Yakovenko A.
|
|
Synthesis dan Anticancer Activity Study of New Bis-1,3-Oxazole-5-Sulfonylamides.
|
ChemMedChem. 2025. e202500921 |
M. Kachaeva, S. Pilyo, D. Hodyna, Y. Shulha, V. Brovarets |
|
Antifungal activity and cytotoxicity of imidazole- and morpholine-based lysosomotropic detergents. |
Innovative Biosystems and Bioengineering. 2025. V. 9, N 1. P. 26-44. |
D. Hodyna, V. Kovalishyn, Yu. Shulha, O. Trokhimenko, O. Aksenovska, S. Rogalsky, L. Metelytsia |
|
Practical One-Pot Four-Step Synthesis of Isocoumarin-3-Carboxylic Acids.
|
Synlett. 2025. |
Vasylyshyn R., Demydchuk B., Kovalenko S., Matiychuk V., Shishkina S., Lukin O., Karpun Y., Shivanyuk A., Fetyukhin V. |
|
Synthesis of 2-R-5-amino-4-(1H-tetrazol-5-yl)-1,3-oxazoles from 2-R-5-amino-1,3-oxazole-4-carbonitriles. |
Current Chemistry Letters. 2025. V. 14 N 1. Р. 233-238 |
Shablykina O., Herasymov E., Kozytskyi A. |
|
Bicyclic Isosteres of Pyridine/Piperidine: From Synthesis to Applications.
|
Angewandte Chemie – International Edition. 2025. V. 64, N 46. e202517814 |
Stashkevych O., Kokhalskyi V., Mynak Y., Levterov V., Shablykin O., Pishel I., Mykhailiuk P.K. |
|
Basicity and Lipophilicity of gem‐Difluorinated Saturated Bicyclic Amines: Advanced Building Blocks for Drug Discovery.
|
European Journal of Organic Chemistry. 2025. V. 28, N 40. e202500728 |
Liashuk O.S., Moroz B., Melnykov K.P., Holovach S., Lesyk D., Lesyk Y., Skrypnik D., Holota Y., Borysko P., Filatov A.A., Grygorenko O.O. |
|
Gram-scale synthesis and physicochemical properties of exo- and endo-5,5-difluorooctahydropentalen-2-amines.
|
Journal of Fluorine Chemistry. 2025. V. 287. P. 110484. https://doi.org/10.1016/j.jfluchem.2025.110484 |
Voloshyna O., Moroz B., Melnykov K.P., Holovach S., Lesyk D., Holota Y., Borysko P., Filatov A.A., Grygorenko O.O. |
|
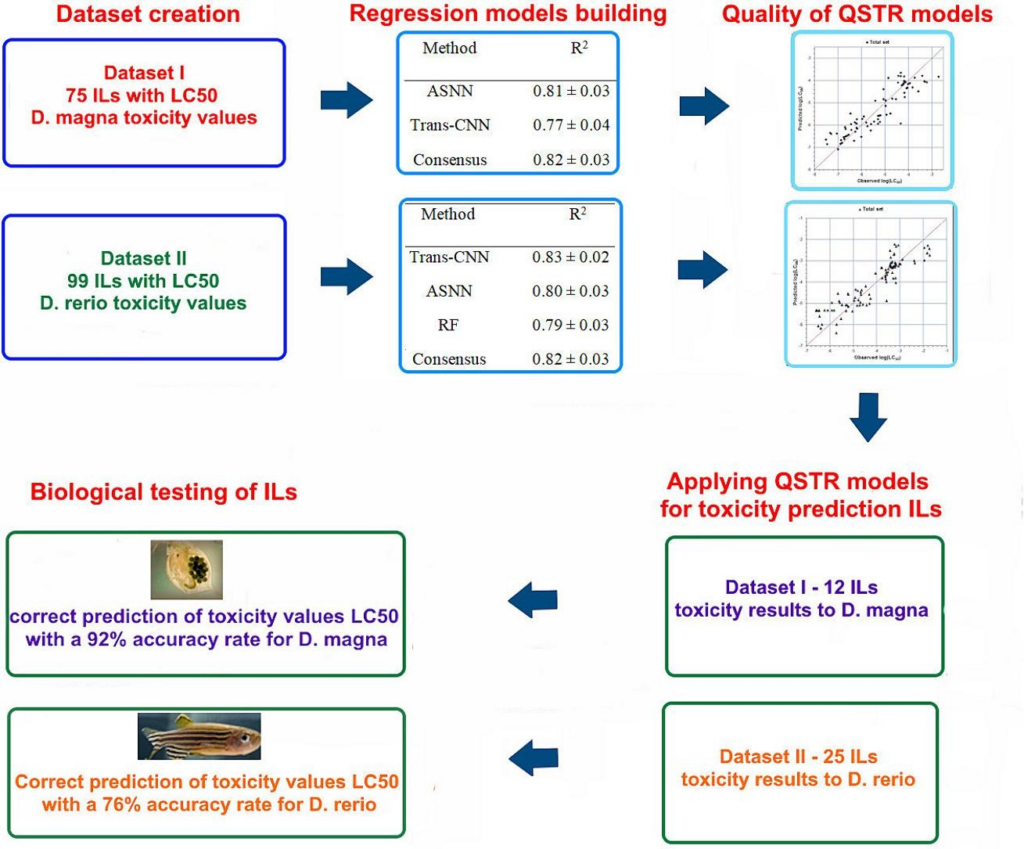
Development of Multi-task QSTR Models for Acute Toxicity Prediction Towards Daphnia magna Using Machine Learning in the OCHEM Platform. |
Medinformatics 2025. V. 2, N 2. P. 93-98. |
V. Kovalishyn, D. Hodyna, L. Metelytsia. |
|
Trifluoroacetyl-Substituted Quinolones as New Antibacterials Against Antibiotic Resistant Strains.
|
Letters in Applied NanoBioScience. 2025. V. 14, N 1. 3. |
D. Hodyna, A. Klipkov, Y. Shulha, N. Shevchenko, M. Kachaeva, V. Kovalishyn, I. Gerus, L. Metelytsia. |
|
In vitro evaluation of antibacterial and antibiofilm activity of new bis-quaternary ammonium compounds based on natural products. |
Current Chemistry Letters. 2025. V. 14. P. 271-278 |
Muzychka L., Hodyna D., Metelytsia L., Smolii O. |
|
Nature-inspired Novel Quaternary Ammonium Compounds: Synthesis, Antibacterial and Antibiofilm Activity.
|
ChemMedChem 2025. V. 20, N 5. e202400807 |
L. Muzychka, D. Hodyna, L. Metelytsia, O. Smolii |
1. Patrylak L.K., Yakovenko A.V., Nizhnik B.O.,Pertko O.P., Melnychuk O.V. Antibacterial Properties of Silver Nanoparticles Deposited on Different Carriers. Springer Proceedings in Physics.In: Nanooptics and Nanoelectronics, Nanobiotechnology, and Their Applications. 2024, V. 312. Springer, Cham. Р. 279-289. https://doi.org/10.1007/978-3-031-67527-0_20
2. M.M. Baran, D.S. Kamenskyh, T.V. Tkachenko, V.G. Burdeinyi, M.M. Filonenko, V.A. Povazhny, V.O. Yevdokymenko. Effect of the low-frequency sound vibrations on the structural and mor-phological properties of the industrial catalysts for the carbon oxides’ hydrogenation. In: Fesenko, O., Yatsenko, L. (eds) Nanomaterials and Nano-composites, and Nanostructure, and Their Applications. NANO 2023. Springer Proceedings in Physics, 2024. Springer, Cham. Chapter 3. Р. 27-39. https://doi.org/10.1007/978-3-031-67519-5_3
3. T. Tkachenko, V. Sokol, O. Haidai, D. Kamenskyh, V. Yevdokymenko. Polyethylene packages and polyethylene terephthalate bottles – a source for chemical syntheses precursors. Chemical Technology and Engi-neering ‒ 2023: Monograph. (Atamanyuk V.M. et al., Eds.). Lviv: Rastr-7, 2023. P. 127-134. ISBN 978-617-8296-99-5
4. Богатиренко В.А., Євдокименко В.О., Каменських Д.С., Ткаченко Т.В., Андрєєва О.В. Основи хімії викопного й альтернативного палива. Навчальний посібник / Київ: Український державний університет імені Михайла Драгоманова, 2024. 117 c.
5. А.І. Вовк. Біоактивні сполуки, нові речовини і матеріали. Київ: Інтерсервіс. 2024. 306 с. ISBN 978-966-999-459-2. https://drive.google.com/file/d/1oB9uBhRoMnED1lq-wAuG7j2y0Xgk3V7E/edit
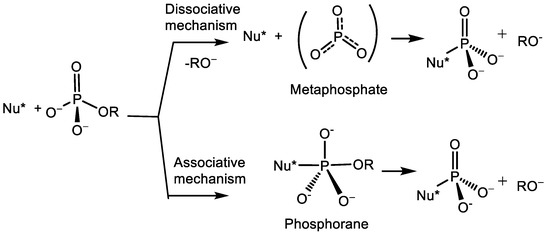
6. Romanenko V.D. Organophosphorus Synthesis beyond P-Cl bond: The Development of Shelf-Stable Reagents for [RP] Transfer. Chemistry. 2024. V. 28, N 19. Р. 1483-1512. DOI: 10.2174/0113852728323258240613061150

7. Kolodiazhnyi O.І, Kolodiazhna A.O. Stereoselective Syntheses of Organophosphorus Compounds. Symmetry. 2024. V.16, N 3. Р. 342. https://doi.org/10.3390/sym16030342
8. O.O. Kolodiazhna, D.V. Prysiazhnuk, A.O. Kolodiazhna, O.I. Kolodiazhnyi. Enzymatic resolution of heterocyclic intermediates for biologically active compound preparation. Phosphorus, Sulfur and Silicon and the Related Elements. 2024. https://doi.org/10.1080/10426507.2024.2367033

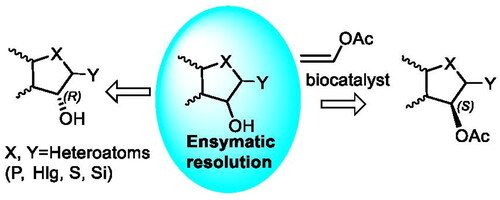
9. Kolodiazhnyi O., Kolodiazhna A., Faiziiev O.,Gurova Y. Enzymatic Deracemization of Fluorinated Arylcarboxylic Acids: Chiral Enzymatic Analysis and Absolute Stereochemistry Using Chiral HPLC. Symmetry. 2024. V.16, N 9. Р. 1150. https://doi.org/10.3390/sym16091150.
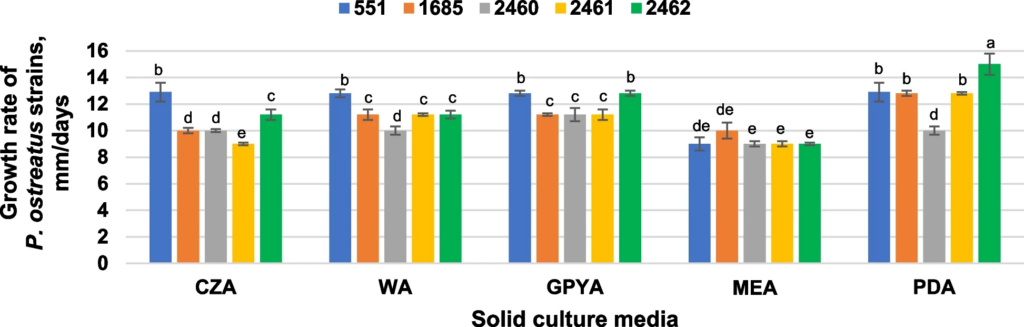
10. Krupodorova T., Barshteyn V., Tsygankova V., Sevindik M., Blume Ya. Strain-specific features of Pleurotus ostreatus growth in vitro and some of its biological activities. BMC Biotechnol. 2024. V. 24, N 9(1). P. 1-14. https://doi.org/10.1186/s12896-024-00834-9
11. Semenyuta I., Kovalishyn V., Hodyna D., Startseva Yu., Rogalsky S., Metelytsia L. New QSTR models to evaluation of imidazolium- and pyridinium-contained ionic liquids toxicity, Computational Toxicology. 2024. V. 30. Р. 100309. https://www.sciencedirect.com/science/article/abs/pii/S2468111324000112

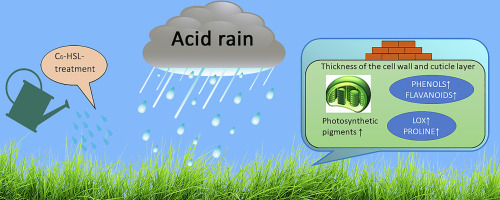
12. Babenko L., Futorna O., Romanenko K., Smirnov O., Rogalsky S., Kosakivska I., kwarek E., Wiśniewska M. Exogenous N-hexanoyl-L-homoserine lactone mitigates acid rain stress effects through modulation of structural and functional changes in Triticum aestivum leaf. Applied Soil Ecology.2024. V. 193. Р. 105151. https://doi.org/10.1016/j.apsoil.2023.105151

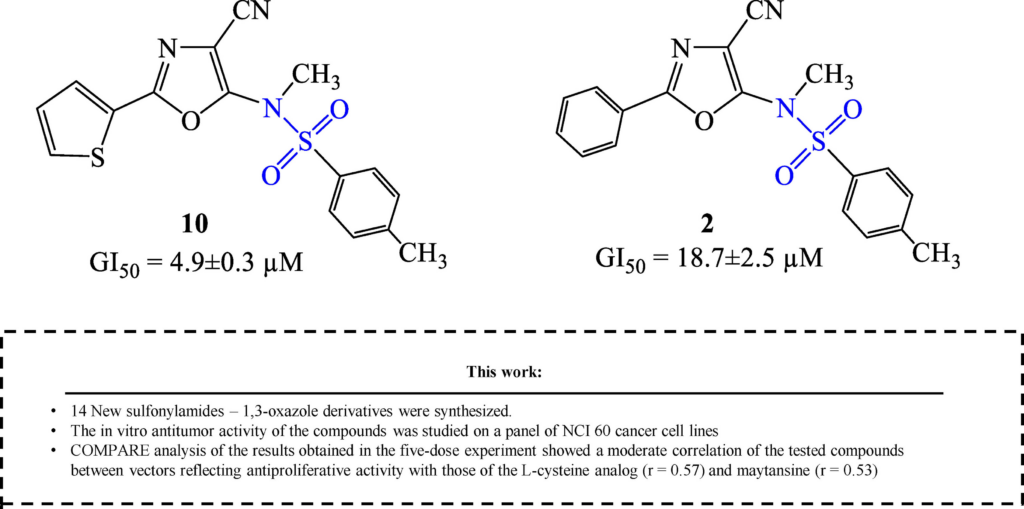
13. O. Severin,S. Pilyo,M. Kachaeva, V. Zhirnov,D. Hodyna,O. Bahrieieva,V. Brovarets. Synthesis, characterization of novel N-(4-cyano-1,3-oxazol-5-yl)sulfonamide derivatives and in vitro screening their activity against NCI-60 cancer cell lines. ChemMedChem, 2024. V. 19, N 5. Р. e202300527. https://doi.org/10.1002/cmdc.202300527

14. Konovalenko A., Shablykin O., Shablykina O., Kozytskyi A., Brovares V. Convenient and versatile method of 8‑amino-6-(2-R-thiazol-4-yl)1,7-naphthyridines synthesis. Curr. Chem. Lett. 2024. V.13, N 1. P. 163-172. http://dx.doi.org/10.5267/j.ccl.2023.7.004
15. Prysiazhniuk K., Datsenko O., Polishchuk O., Shulha S., Shablykin O., Nikandrova L., orbatok K., Bodenchuk I., Borysko P., Shepilov D., Pishel I., Kubyshkin V., Mykhailiuk P. Spiro[3.3]heptane as a saturated benzene bioisostere. Angewandte Chemie. Int. Ed. 2024. V. 63, N 9. Р. e202316557. https://doi.org/10.1002/anie.202316557

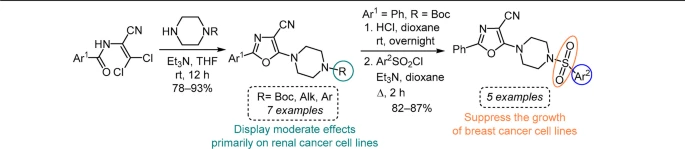
16. Severin O.,Pilyo S., Moskvina V., Shablykina O., Karpichev Y., Brovarets V. Synthesis and in vitro anticancer evaluation of functionalized 5-(4-piperazin-1-yl)-2-aryloxazoles, and 5-(4-arylsulfonyl)piperazine-1-yl)-2-phenoloxazoles. Chem Heterocycl Comp. 2024. V. 60, N 1/2. P. 68-74. https://doi.org/10.1007/s10593-024-03295-2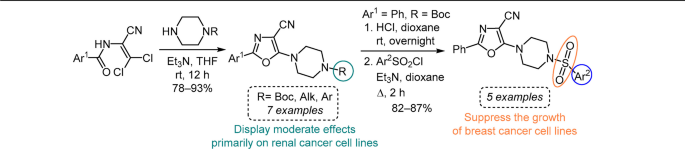
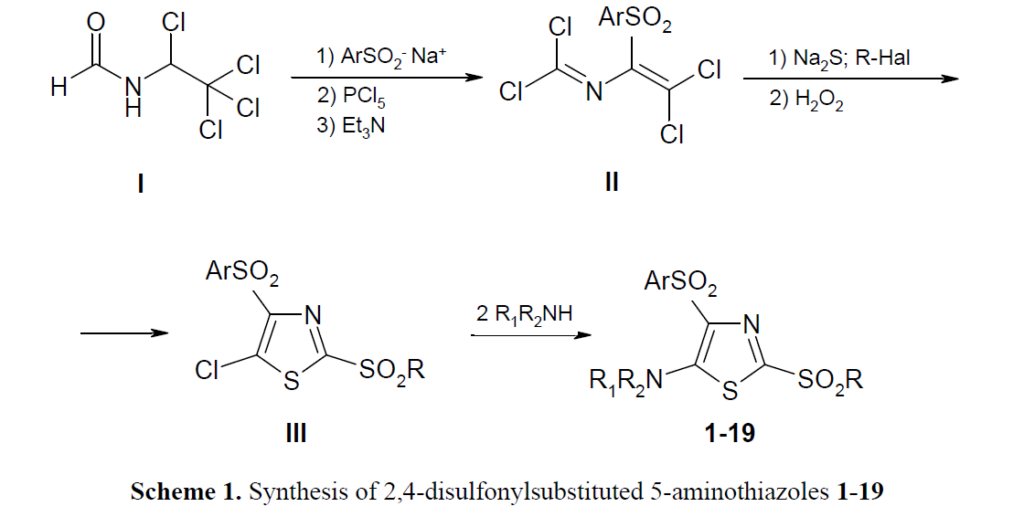
17. Zyabrev V., Demydchuk B., Pilyo S., Zhirnov V., Liavynets O., Brovarets V. Synthesis, characterization, and in vitro anticancer evaluation of 2,4-disulfonyl-substituted 5-aminothiazoles. Curr Chem Lett. 2024. V. 13, N 3. P. 557-568. https://doi.org/10.5267/j.ccl.2024.2.003
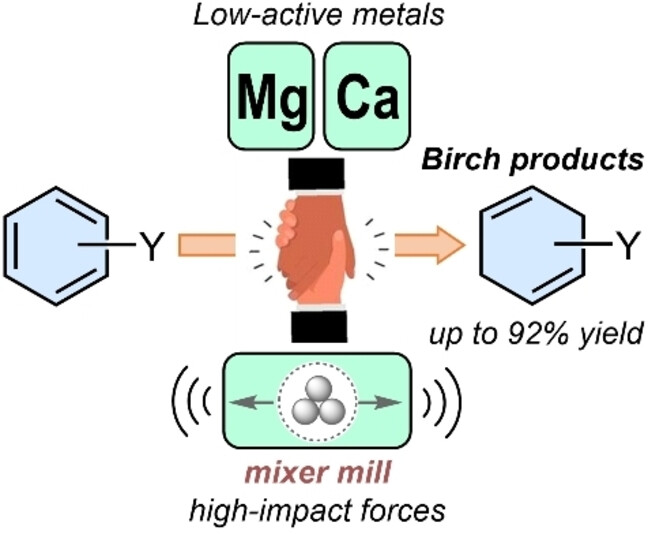
18. Nallaparaju J.V., Satsi R., Merzhyievskyi D., Jarg T., Aav R., Kananovich D.G. Mechanochemical birch reduction with low reactive alkaline earth metals. Angew Chem Intern Ed. 2024. V. 63, N 20. Р. e202319449. https://doi.org/10.1002/anie.202319449

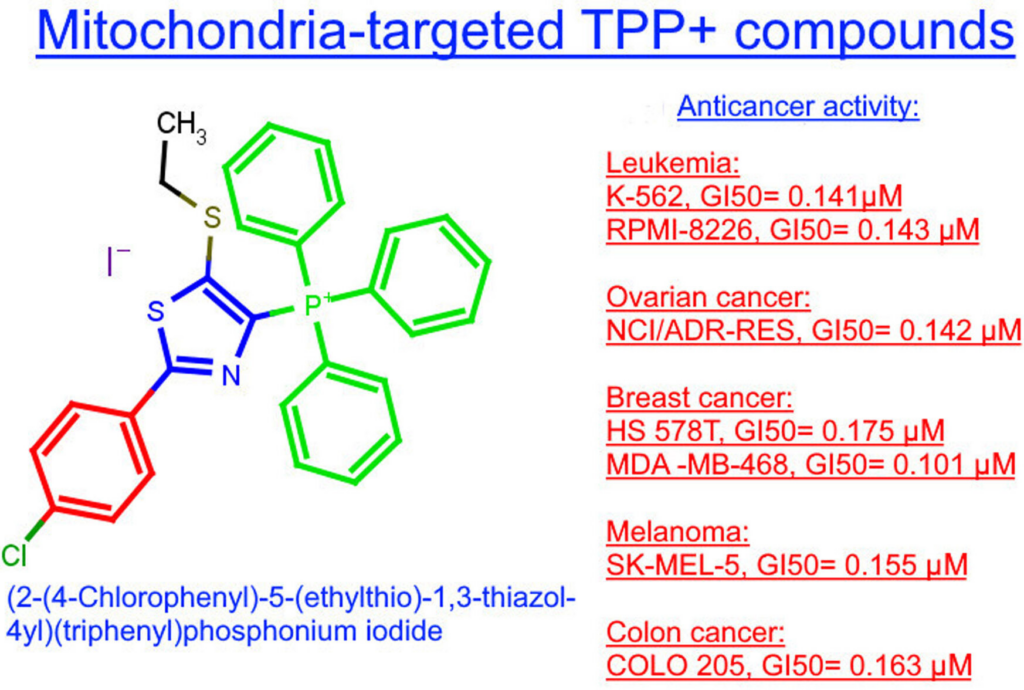
19. Semenyuta I., Golovchenko O., Bagreeva O., Vydzhak R., Zhirnov V., Brovarets V. Synthesis, characterization, in vitro anticancer evaluation, ADMET properties, and molecular docking of novel 5-sulfonyl substituted (thiazol-4-yl)posphonium salts. ChemMedChem. 2024. E202400205. P. 1-11. https://doi.org/10.1002/cmdc.202400205

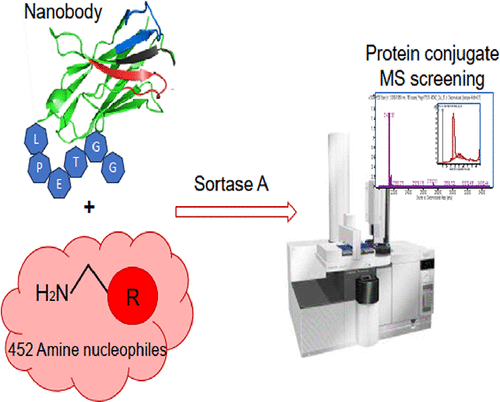
20. Bondarchuk T., Vaskiv D., Zhuravel E., Shyshlyk O., Hrynyshyn Ye., Nedialko O., okholenko O., Pohribna A., Kuchuk O., Brovarets V., Zozulya S. Synthetic amine lincers for efficient sortagging. Bioconjugate Chem. 2024. V. 35, N 8. P. 1172-1181. https://pubs.acs.org/doi/abs/10.1021/acs.bioconjchem.4c00143

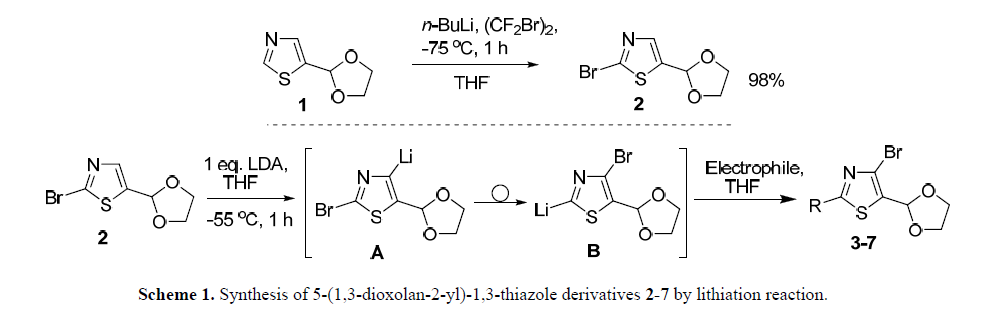
21. Sinenko V.O., Los O.V., Potikha L.M., Brovarets V.S. Functionalized 1,3-thiazoles by combined halogen dance. Curr Chem Lett. 2024. V. 13, N 4. P. 695-706. https://www.growingscience.com/ccl/Vol13/ccl_2024_22.pdf
22. Sosnovich B.D., Timokhin O.S., Kucha O.V., Demianyuk N.Y., Hys D.V., Zvarych .A., Smolii O.B., Vashchenko B.V., Grygorenko O.O. Synthesis and enzymatic resolution of novel analogues of alicyclic and heterocyclic mandelic acid derivatives. Tetrahedron. 2024. V. 161. P. 134068. https://doi.org/10.1016/j.tet.2024.134068

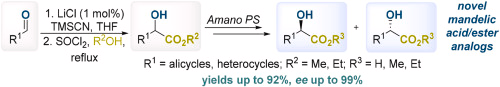
23. Shablykin O.V., Chumachenko S.A., Konovalenko A.S., Shablykina O., Shishkina S.V., Kozytskyi A.V., Brovarets V.S. Interactions of 3-acylisocoumarines: a simple route to 3,4-dihydro-6H-pyrazino[1,2-b]isoquinolin-6-ones and their hydrogenated derivatives. Chemistry Select. 2024. V. 9, N 40. 2024. Р. e202403740. https://doi.org/10.1002/slct.202403740

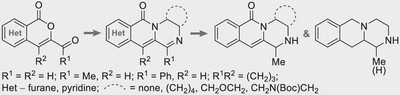
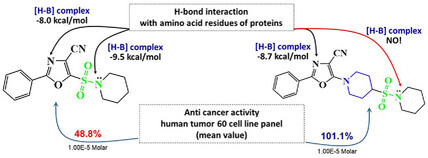
24. Obernikhina N., Severin O., Pilyo S., Kachaeva M., Kachkovsky O., Kozachenko O., Brovarets V., Bodachivskii Yu. A comparative in vitro and in silico study of the anticancer action of 1,3-oxazol-5-sulfonylamides and new 1-(1,3-oxazol-5-yl)piperidine-sulfonylamides. Chemistry Select. 2024. V.9, N 41. Р. e202403531. https://doi.org/10.1002/slct.202403531

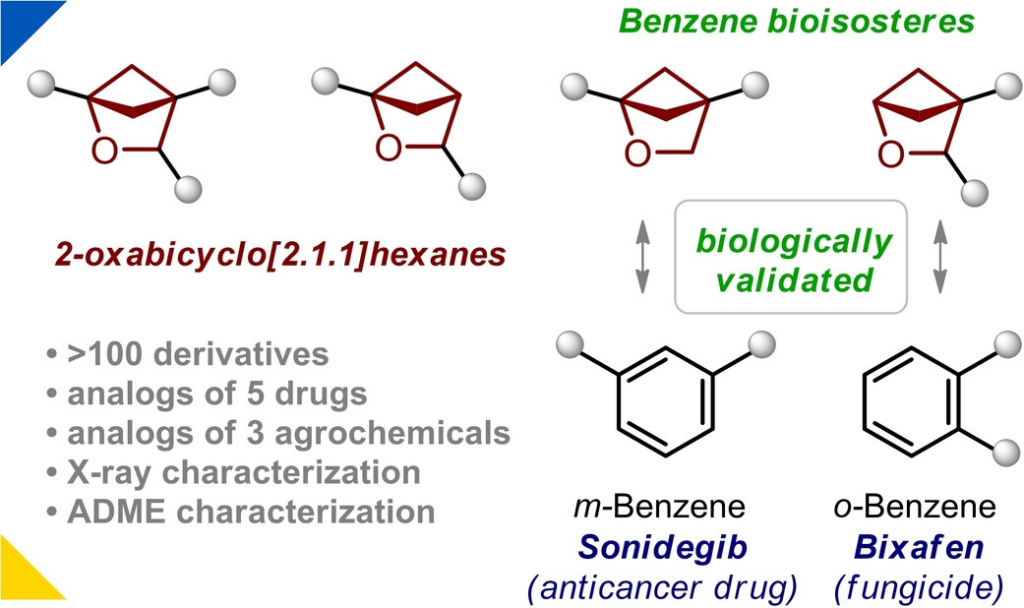
25. Levterov V.V., Panasiuk Ya., Shablykin O., Stashkevych O., Sahun K., Rassokhin A., Sadkova I., Lesyk D., Anisiforova A., Holota Yu., Borysko P., Bodenchuk I., oloshchuk N.M., Mykhailiuk P.K. 2-Oxabicyclo[2.1.1]hexanes: synthesis, properties, and validation as bioisosteres of ortho- and meta-benzenes. Angew. Chem. Int. Ed. 2024. V. 63, N 19. Р. e202319831. https://doi.org/10.1002/anie.202319831

26. Myshko A.S., Mrug G.P., Bondarenko S.P., Kondratyuk K.M., Kobzar O.L., Buldenko .M., Kozytskiy A.V., Vovk A.I., Frasinyuk M.S. Trapping of thermally generated ortho– and para-quinone methides by imidazoles and pyrazoles: a simple route to green synthesis of benzopyrone-azole hybrids and their evaluation as α-glucosidase inhibitors. RSC Adv. 2024. V.14. P. 27809-27815. https://doi.org/10.1039/d4ra05230g
27. Govor E. V., Naumchyk V., Nestorak I., Radchenko D.S., Dudenko D., Moroz Yu.S., Kachkovsky O.D., Grygorenko O.O. Generation of multimillion chemical space based on the parallel Groebke–Blackburn–Bienaymé reaction. Beilstein J. Org. Chem. 2024. V. 20. P. 1604-1613. https://doi.org/10.3762/bjoc.20.143
28. Gaponov A.M., Ryzhkova A.S., Pavlenko O.L., Dmytrenko O.P., Kulish M.P., Lesiuk .I., Kolomys O.F., Obernikhina N.V., Kachkovsky O.D. Spectral features of films of bovine serum albumin with thiochrome. Mol. Cryst. Liquid Cryst. 2024. https://doi.org/10.1080/15421406.2024.2355394
29. Shablykin O., Herasimov E., Shablykina O., Kozytsky A. Synthesis of 2-R-5-amino-4-(1H-tetrazol-5-yl)-1,3-oxazoles from 2-R-5-amino-1,3-oxazole-4-carbonitriles. Curr. Chem. Lett. 2025. V.14, N 1. P. 233-238. https://doi.org 10.5267/j.ccl.2024.6.003
30. Golovchenko O.V., Brusnakov M.V., Shabelko Yu.O., Brovarets V.S., Vydzhak R.M., Bahrieieva O.S., Potikha L.M., Shishkina S.V. Synthesis and properties of methanesulfonyl derivatives of diethyl esters of 5-(hydroxyalkylamino)-1,3-oxazol-4-ylphosphonic acids. Phosph. Sulph. Silicon and Relat. Elem. 2024. V. 199, N 1. P. 71-81. Doi: 10.1080/10426507.2023.2251639

31. Hlibov E.K., Gorbulenko N.V., Moskvina V.S., Shablykina O.V., Shokol T.V., Kozytskyi A.V., Khilya V.P. Modified neoflavones based on 7-hydroxyneoflavone-6-enamino ketone and 7-hydroxy-3-hetarylbenzopyran-2- and 4-ones Mannich bases and their recyclization.. Chem. Nat. Compd. 2024. V. 60, N 3. P. 223-228. https://doi.org/10.1007/s10600-024-04293-8
32. Gaponov A.M., Pavlenko O.L., Dmytrenko O.P., Neimash V.B., Kachkovsky O.D. Spectral Properties of Thin Films of Squaraine Dyes, Deposited on Silver and Gold Nanoparticles. Springer Proceedings in Physics. 2023. V. 297. P. 339-354. https://doi.org/10.1007/978-3-031-42708-4_22
33. Gryniukova A., Borysko P., Myziuk I., Alieksieieva D., Hodyna D., Semenyuta I., ovalishyn V., Metelytsia L., Rogalsky S., Tcherniuk S. Anticancer activity features of imidazole-based ionic liquids and lysosomotropic detergents: in silico and in vitro studies. Molecular Diversity 2024. https://doi.org/10.1007/s11030-023-10779-4
34. Demchenko S., Sukhovieiev V., Golovchenko O., Sukhovieiev J., Yarmoluk S., Demchenko A. Syntheses and evaluation of novel 3-hydroxy-1,3-diaryl-2,3,5,6,7,8-hexahydroimidazo[1,2-a]pyridine-1-ium bromides as potential anticancer agents. Pharmacia. 2024. V.71. P.1-10. https://doi.org/10.3897/pharmacia.71.e135992
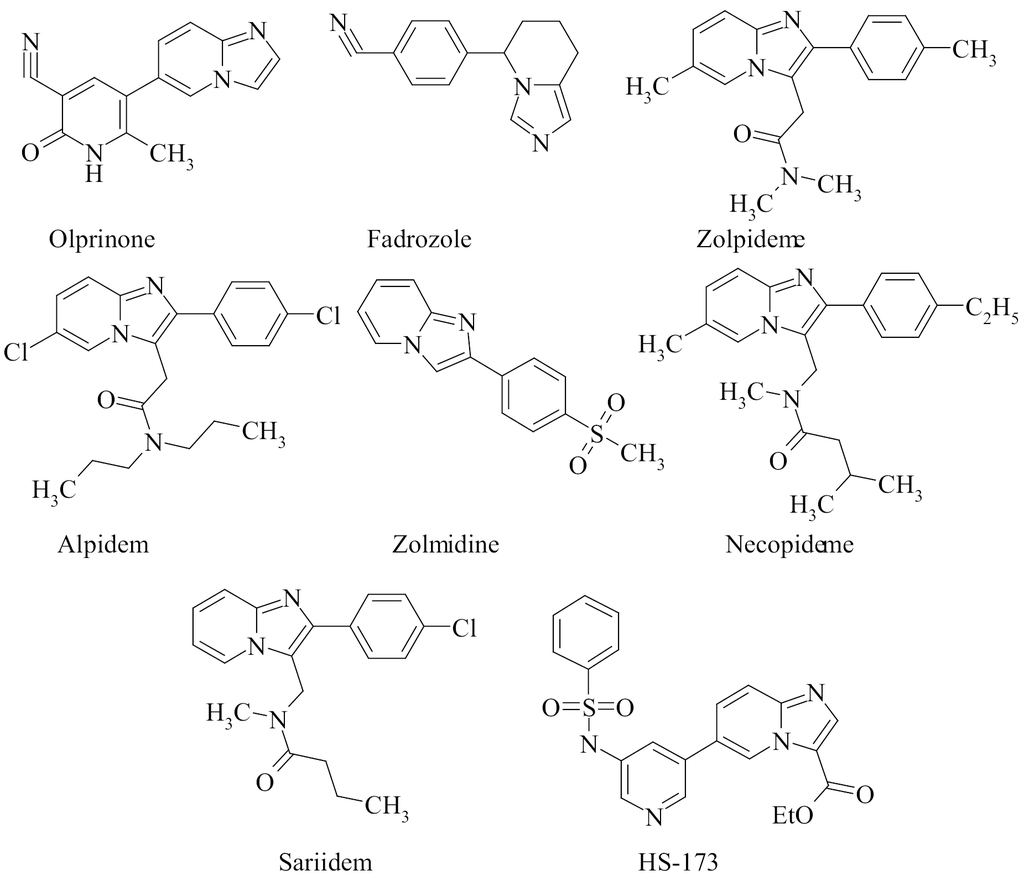
35. Shablykin O.V., Brovarets V.S., Shablykina O.V. Recyclization of 5-aminooxazoles as a route to new functionalized heterocycles. Chem. Record: Spec. 2024. V. 24, N 2. Р. e202300264. https://doi.org/10.1002/tcr.202300264
36. Semenyuta I., Hodyna D., Kovalishin V., Demydchuk B., Pilyo S., Metelytsia L. 5-Amino-4-cyano-1,3-oxazoles as new antibacterials against antibiotic-resistent Escherichia coli strains. Proceedings “Scientific practice: Modern and classical research methods”. May 26, 2023. Boston, USA. P. 117-121. https://doi.org/10.36074/logos-26.05.2023
37. Kobzar O., Beiko A., Merzhyievskyi D., Shablykin O.,Brovarets V.,Tanchuk V., Vovk A. Design, synthesis, and xanthine oxidase inhibitory activity of 4‐(5‐aminosubstituted‐4‐cyanooxazol‐2‐yl) benzoic acids. ChemMedChem. 2024. Р. e202400478. DOI: 10.1002/cmdc.202400478

38. Parkhomenko Y.M.,Vovk A. I.,Protasova Z. S., Pylypchuk S.Y., Chorny S.A., Pavlova O.S., Pylypchuk S.Yu., Stepanenko S.P. Thiazolium salt mimics the non-coenzyme effects of vitamin B1 in rat synaptosomes. Neurochemistry International. 2024. V. 178. Р. 105791. DOI: 10.1016/j.neuint.2024.105791
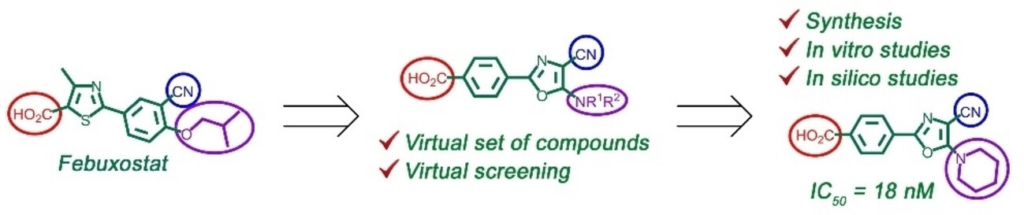
39. Myshko A.S., Mrug G.P., Bondarenko S.P., Demydchuk B.A., Kobzar O.L., Buldenko V.M., Vovk A.I., Frasinyuk M.S. Divergent synthesis of novel 3 (5)‐aminoazole–benzopyrone hybrids and their evaluation as α‐glucosidase inhibitors. ChemMedChem. 2024. Р. e202400525. https://doi.org/10.1002/cmdc.202400525

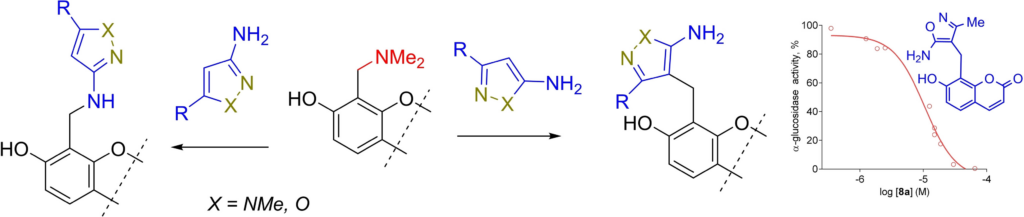
40. Semenyuta I.,Los O.,Sinenko V.,Zhirnov V.,Potikha L.,Kobzar O.,Brovarets V. Design, synthesis, and antitumor potential of new thiazole-contained 5-fluoro-2-oxindole derivatives as sunitinib analogues. Current Medicinal Chemistry. 2024. doi: 10.2174/0109298673346427241016100726
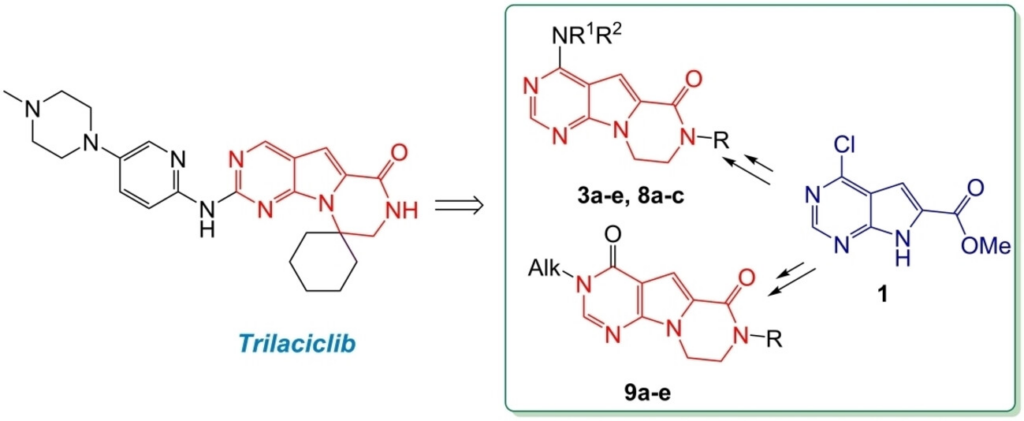
41. Muzychka L., Muzychka O.,Smolii O. Synthesis and acetylcholinesterase inhibitory activity of novel trilaciclib analogs. Chemistry and Biodiversity. 2024 Р. 02401874. https://doi.org/10.1002/cbdv.202401874

42. Muzychka L.V., Humeniuk N.I., Boiko I.O., Vrynchanu N.O., Smolii O.B. Synthesis and in vitro evaluation antibacterial and antibiofilm activities of novel triphenylphosphonium-functionalized substituted pyrimidines. Chemical Biology & Drug Design. 2024. V. 103, N 2. Р. e14483. DOI: 10.1111/cbdd.14483
43. Muzychka L.V., Humeniuk N.I., Boiko I.O.,Vrynchanu N.O., Smolii O.B. Synthesis and antibiofilm activity of novel 1,4-dihydropyrido [1,2-а]pyrrolo[2,3-d]pyrimidine-2-carboxamides. Biopolymers&Cell. 2024. V. 40, N 1. Р. 68-80 http://dx.doi.org/10.7124/bc.000AAB
44. Dubina T.F., Kosarevych A.V., Kucher O.V., Sosunovych B.S., Smolii O.B., Vashchenko B.V., Grygorenko O.O. Synthesis and reactions of novel imidazo[4,5-b]pyridine building blocks. Chemistry of Heterocycllic Compounds. 2024. V. 60. Р. 175-182. https://doi.org/10.1007/s10593-024-03315-1
45. Vaskevych A., Shishkina S., Dekhtyar M.,Smolii O.,Vovk M. Access to Seleno-Functionalized Thiazolo[3,2-a]Pyrimidinones and Pyrimido[2,1-b][1,3]Thiazinones via Selenocyclization of 2-Alkenylthiopyrimidinones and Their Fused Analogs. Chemistry Select. 2024. V. 9. Р. e202403699. https://doi.org/10.1002/slct.202403699

46. S.I. Vdovenko, I.I. Gerus, M. Pagacz-Kostrzewa, M. Wierzejewska. Comparison of kinetic and thermodynamic parameters of reaction of individual conformers of α‑substituted β‑ethoxyvinyl trifluoromethyl ketones with secondary amines. Reaction Kinetics, Mechanisms and Catalysis. 2024. V. 137, N 2. Р. 1-18. DOI:10.1007/s11144-024-02578-1
47. Q. Wang, Y. Bian, G. Dhawan, W. Zhang, A.E. Sorochinsky, A. Makarem, V. A. Soloshonok, J. Han. Fluorine-containing drugs approved by the FDA in 2023. Chinese Chemical Letters. 2024. V. 35. Р. 109780. doi: 10.1016/j.cclet.2024.109780

48. Logvinenko I.G., Sadkova I.V., Tolmachova N.A., Shishkina S.V., Daniliuc K.G., Haufe G., Kondratov I.S. 4-Trifluoromethoxy proline: synthesis of stereoisomers and lipophilicity study. Org. Biomol. Chem. 2024. V. 22. Р. 7982-7988. DOI https://doi.org/10.1039/D4OB00688G
49. Pahl A., Grygorenko O.O., Kondratov I.S., Waldmann H. Identification of readily available pseudo-natural products. RSC Med. Chem. 2024. V. 15. Р. 2709-2717. https://pubs.rsc.org/en/content/articlelanding/2024/md/d4md00310a
50. F. Liu, A.L. Kaplan, J. Levring, J. Einsiedel, S. Tiedt, K. Distler, N.S. Omattage, I.S. Kondratov, Y.S. Moroz, H.L. Pietz, J.J. Irwin, P. Gmeiner, B.K. Shoichet, J. Chen. Structure-based discovery of CFTR potentiators and inhibitors. Cell. 2024. V. 187, N 14. P. 3712-3725. https://pubmed.ncbi.nlm.nih.gov/38810646/
51. P. Janssen, F. Becker, F.T. Füsser, N. Tolmachova, T. Matviiuk, I. Kondratov, M.S. Weiss, D. Kümmel, O. Koch. Design and Crystallographic Screening of a Highly Sociable and Diverse Fragment Library Towards Novel Antituberculotic Drugs. ChemRxiv. 2024. doi: 10.26434/chemrxiv-2024-rpst3-v2
52. Kolesnikov Ya.S., Kretynin S.V., Filepova R., Dobrev P.I., Martinec J., Kravets V.S. Polyamines metabolism and their biological role in plant cells: what do we really know? Phytochem. Rev. 2024. V. 23. P. 997-1026 . DOI:10.1007/s11101-024-09913-3
53. Voloshyna Yu.,Pertko O.,Yakovenko A., Povazhnyi V.,Patrylak L. Metal-containing zeolite composites with separated phases as catalysts for hydroisomerization of linear hexane. J. Porous Mater. 2024. V. 31, N 3. P. 1029-1041. https://doi.org/10.1007/s10934-024-01584-x
54. O. Pertko,Yu. Voloshyna,L. Patrylak,A. Yakovenko. Oxidative CO2 dehydrogenation of butane on microspherical zeolite-containing composites based on kaolin. ChemRxiv,Preprints. 2024.20 May 2024. https://doi.org/10.26434/chemrxiv-2024-xqcjg
55. A. Yakovenko,L. Patrylak,Yu. Voloshyna,O. Pertko,V. Povazhnyi. Ni/Pd-containing pentasils in n-hexane micropulse hydrocracking and aromatization. Research Square, Preprints. 2024.08 May 2024. https://doi.org/10.21203/rs.3.rs-4375758/v1
56. L. Patrylak,Yu. Voloshyna,O. Pertko,A. Yakovenko. n-Alkanes hydroisomerization on Ni-HMFI zeolites of different preparation methods. Research Square, Preprints. 2024.01 April 2024. https://doi.org/10.21203/rs.3.rs-4187059/v1
57. L. Patrylak,S. Konovalov,S. Zubenko,A. Yakovenko, D. Davitadze,O. Pertko. Fatty Acid Ethyl Esters as Biodiesel Fuel: Product Quality and Efficiency of Various Purification Techniques. SRNN Preprints, 2024. 3 June 2024. http://dx.doi.org/10.2139/ssrn.4852240
58. Yu.Voloshyna,O. Pertko,A. Yakovenko,V. Povazhnyi, L. Patrylak. Metal-containing zeolite composites with separated phases as catalysts for hydroisomerization of linear hexane. Research Square, Preprints. 2024.12 March 2024. https://doi.org/10.21203/rs.3.rs-3796081/v1
59. Povazhnyi V.A., Voloshyna Y.G., Pertko O.P., Melnychuk O.V., Kontsevoi A.L Enhancing the thermal stability of nanostructured carbonaceous materials using an improved method of template synthesis. Appl. Nanosci.2023. V. 13, N 12. Р. 7491-7499. https://doi.org/10.1007/s13204-023-02908-0
60. Hutsul,I. Ivanenko, L. Patrylak. Photocatalytic degradation of azo dyes by ZnO/zeolite composite under static conditions. Appl. Nanosci.2023. V. 13, N 12. Р. 7601-7609. https://doi.org/10.1007/s13204-023-02950-y
61. L. Patrylak,S. Konovalov,S. Zubenko,A. Yakovenko,D. Davitadze,O. Pertko. Fatty acid ethyl esters as biodiesel fuel: product quality and efficiency of various purification techniques. Chemistry Journal of Moldova. 2024. https://doi.org/10.19261/cjm.2024.1236
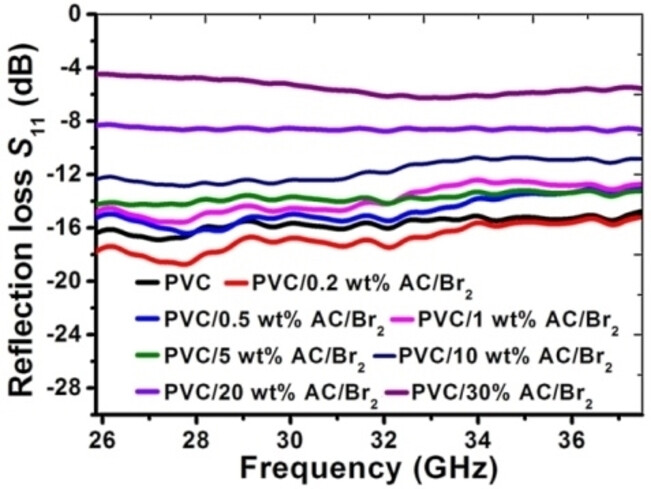
62. L.M. Grishchenko, D.O. Zhytnyk, I.P. Matushko, V.E. Diyuk, Yu.V. Noskov. V.Yu. Malyshev, V.A. Moiseienko, O.Yu. Boldyrieva, V.V. Lisnyak. Microwave properties of composite films based on polyvinyl chloride and brominated activated carbon. ChemistrySelect. 2024. V. 9, №18. Р. e202400432. https://doi.org/10.1002/slct.202400432

63. Tsygankova V.A., Andrusevich Ya.V., Vasylenko N.M., Kopich V.M., Popilnichenko S.V., Pilyo S.G., Brovarets V.S. Auxin-like and cytokinin-like effects of new synthetic pyrimidine derivatives on the growth and photosynthesis of wheat. J. Plant Sci. Phytopathol. 2024. V. 8, N 1. P. 15-24. DOI: https://dx.doi.org/10.29328/journal.jpsp.1001126
64. Tsygankova V.A., Vasylenko N.M., Andrusevich Ya.V., Kopich V.M., Solomyannyi R.M., Pilyo S.G., Bondarenko O.M., Popilnichenko S.V., Brovarets V.S. New Wheat Growth Regulators Based On Thioxopyrimidine Derivatives. Int. J. Med. Biotechnol. Genetics. 2024. S1:02:004:23-30. https://scidoc.org/IJMBG-2379-1020-S1-02-004.php
65. Zhirnov V., Shablykin O., Chumachenko S., Kornii Y., Keith K.A., Harden E.A., Hartline C.B., James, S.H., Kobzar O., Kovalishyn V., Vovk A., Brovarets V. In vitro activity of novel 4-iminohydantoin sulfamide derivatives against human cytomegalovirus, Chem. Pap. 2024. V. 78, N 1. Р. 133-140. DOI:10.1007/s11696-023-03038-1
66. Kovalishyn V., Severin O., Kachaeva M., Kobzar O., Keith K., Harden E., Hartline C., James S., Vovk A., Brovarets V. In Silico Design and Experimental Validation of Novel Oxazole Derivatives Against Varicella zoster virus. Molecular biotechnology. 2024. V. 66, N 4. Р. 70 7-717. DOI: 10.1007/s12033-023-00670-w
67. Severin O.O., Kachaeva M.V., Pilyo S.G., Kovalishyn V.V.,Keith K.A., Harden E.A., Hartline C.B., James S.H., Zhirnov V.V., Brovarets V.S. Synthesis, Characterization, and Study of Anti-HPV Activity and Cell Cytotoxicity of Novel 1,3-Oxazole-4Carbonitrile and 4-Sulfonylamide-5-Phenyl-1,3-Thiazole Derivatives in Vitro. Letters in Applied NanoBioScience. 2024. V. 13, N 2. Р. 89. DOI:10.33263/lianbs132.089
68. Zubova G., Melnyk H., Zaets I., Sergeyeva T., Havryliuk O., Rogalsky S., Khirunenko ., Zaika L., Ruban T., Antonenko S., Kozyrovska N. Halochromic Bacterial Cellulose/Anthocyanins Hybrid Polymer Film with Wound-Healing Potential. Polymers. 2024. V.16, N 16. Р. 2327. https://doi.org/10.3390/polym16162327
69. Kobrina L., Boiko V., Shtompel V., Hudzenko N., Rogalsky S., Frasinyuk M., Kozitskiy A., Riabov S. Inclusion complex of ionic liquid1-dodecylpyridinium tetrafluoroborate with sulfobutyl ether-β-cyclodextrin: preparation and characterization. Journal of Molecular Structure. 2024. V. 1309. Р. 138137. https://doi.org/10.1016/j.molstruc.2024.138137

70. Tsygankova V.A., Andrusevich Ya.V., Vasylenko N.M., Kopich V.M., Pilyo S.G., Solomyannyi R.M., Popilnichenko S.V., Bondarenko O.M., Brovarets V.S. The use of thioxopyrimidine derivatives for the regulation of vegetative growth of wheat. Journal of Medicinal Botany. 2024. V. 8.P. 1-7. DOI: https://doi.org/10.25081/jmb.2024.v8.8918.
71. Tsygankova V.A., Andrusevich Ya.V., Vasylenko N.M., Kopich V.M., Solomyannyi R.M., Popilnichenko S.V., Kozachenko O.P., Pilyo S.G.,Brovarets V.S. The use of thioxopyrimidine derivatives as new regulators of growth and photosynthesis of barley. J. Plant. Sci. Phytopathol. 2024. V. 8, N 2. P. 090-099. DOI: https://dx.doi.org/10.29328/journal.jpsp.1001139.
72. Tsygankova V., Andrusevich Ya., Kopich V., Vasylenko N., Solomyannyi R., Popilnichenko S., Kachaeva M., Kozachenko O., Pilyo S., Brovarets V. Wheat growth in the vegetative phase under the regulatory effect of furopyrimidine derivatives. The scientific heritage. 2024. N 140. P. 3-12. DOI: 10.5281/zenodo.12720609
73. Tsygankova V., Vasylenko N., Andrusevich Ya.., Kopich V., Kachaeva M., Popilnichenko S., Kozachenko O., Pilyo S., Brovarets V. Application of thienopyrimidine derivatives as new eco-friendly wheat growth regulators. Sciences of Europe. 2024. N 146.P. 8-18. DOI: 10.5281/zenodo.13267799
74. V. Bohatyrenko, D. Kamenskyh, M. Jafarov, T. Tkachenko, V. Yevdokymenko. Investigation of oxidation-reduction processes of nickel hydroxides precipitation and their carbothermical reduction. Phys. Chem. Chem. Phys. 2024. Advance Article. https://doi.org/10.1039/D4CP03077J
75. V.A. Bohatyrenko, D.S. Kamenskyh, M.A. Jafarov, T.V. Tkachenko, V.O. Yevdokymenko. Synthesis of nickel nano-particles with magnetic properties using the car-bothermy method. Journal of Physics & Space Sciences. 2024. V. 1, N 2. P. 17-30. ISSN: 3006-6123 (ONLINE)
76. Rogalsky S., Moshynets O., Dzhuzha O., Tarasyuk O., Hubina A., Darbut A.M., Lobko Y., Morozovska I., Protasov O. Bardeau J.-Fr. Preparation and characterization of antifouling coating based on commercial alkyd paint modified with hydrophobic cationic biocide. Journal of Coatings Technology and Research. 2024. V. 21, N 3. Р. 939-953. Doi:10.1007/s11998-023-00862-8
77. Gaponov A. M., Pavlenko O. L., Dmytrenko O. P., Kulish M. P., Ryzhkova A. S., Lesiuk A.I., Obernikhina N.V., Łuszczyńska B., Kachkosky O. D. Molecular heteroassociation in films of thiochrome and tryptophan. Mol. Cryst. Liquid Cryst. 2024. V. 768, N 3. P. 71-77. https://doi.org/10.1080/15421406.2023.2257515
78. L.M. Grishchenko, D.О. Zhytnyk, I.P. Matushko, Yu.V. Noskov, V.A. Moiseienko, V.V. Klepko, O.Yu. Boldyrieva, Yu.А. Len, N.A. Atamas, V.A. Marianovskyi, O.V. Mischanchuk, V.V. Lisnyak. Facile preparation of polyvinyl chloride/activated carbon thin-film composites and study of their microwave absorption at Ka-band frequencies. Molecular Crystals and Liquid Crystals. 2024. V. 768, № 8. P. 139-149. https://doi.org/10.1080/15421406.2024.2348193
79. М. Kharkhota, М. Kharchuk, А. Kharchuk, G. Grabova, Yu. Noskov, R. Linnik, А. Makeiev, L. Avdieieva. Physico-chemical properties of Priestia endophytica UCM B-5715 fluorescent pigments. Biochemical and Biophysical Research Communications. 2024. V. 741. Р. 151040. DOI: 10.1016/j.bbrc.2024.151040
80. O.V. Pavliuk, M.M. Baran, Ye.V. Sheludko, Yu.I. Bogomolov. Heterocyclic inhibitors of autoxidation of hydrocarbons and alcohols. Functional Materials. 2024. V. 31, No 1.Р. 67-75. http://dx.doi.org/10.15407/fm31.01.67
81. I.A. Opeida , O.A. Velichko, Ye.V. Sheludko, A.V. Pavliuk, R.B. Sheparovych, V.E.Sheludko, M.N. Baran. Metal complex catalysis of initiated oxidation of hydrocarbons and alcohols: features of inhibition. nctional Materials. 2024. V. 31, No.2. Р. 269-275. http://dx.doi.org/10.15407/fm31.02.269
82. A.S. Avksentiev, V.Sh. Saberov, G.F. Rayenko, A.B. Ryabitsky, E.V. Polunkin, S.M. Pleskun, N.I. Korotkikh. Catalysis of the Transesterification Reaction of Alkyl Benzoates and Vegetable Oils by Carbonates, Carbene, and Anionite. Theoretical and Experimental Chemistry. 2024. V. 59. Р. 427–433. https://doi.org/10.1007/s11237-024-09802-y
83. T.V. Tkachenko, O.O. Haidai, D.S. Kamenskyh, Y.V. Sheludko, O.V. Pavliuk, V.O. Yevdokymenko. Physicochemical characteristics of microcrystalline cellulose from switchgrass (Panicum virgatum L.) obtained in the presence of a solid catalyst. Chemistry, Physics and Tech-nology of Surface. 2024. V. 15, No 1. P. 57-66. https://doi.org/10.15407/hftp15.01.057
84. V.A. Bohatyrenko, V.A. Nesterovskyi, D.S. Kamenskyh, V.O. Yevdokymenko, T.V. Tkachenko, O.V. Andreieva. Природа активних центрів поверхні сапонітів ташківського родовища України. Chemistry, Physics and Tech-nology of Surface. 2024. V. 15, No 2. P. 183-199. https://doi.org/10.15407/hftp15.02.183
85. Б.В. Коріненко, В.О. Євдокименко, А.П. Ранський, О.А. Гордієнко, Р.В. Коріненко. Альтернативна енергетика. Повідомлення ІІІ. Удосконалена технологія піролізної переробки суміші полімерних відходів. Вісник Вінницького політехнічного інституту. 2024. № 2. С. 25-32 https://doi.org/10.31649/1997-9266-2024-173-2-25-32
86. L.O. Barybina, T.V. Tkachenko, O.O. Haidai, B.V. Korinenko, D.S. Kamenskyh, Y.V. Sheludko, V.A. Povazhny, V.A. Bohatyrenko, S.V. Ruban, V.O. Yevdokymenko. Structural and morphological features of microcrystalline сellulose from industrial hemp hurd. Chemistry, Physics and Tech-nology of Surface. 2024. V. 15, No 4. P. 524-533. https://doi.org/10.15407/hftp15.04.524
87. Morozovska I.O., Rogalsky S.P., Dzhuzha O.V., Tarasyuk O.P., Protasov O.O. Zooperiphyton on anti-fouling coatings and changes in its coenotic structure. Hydrobiological Journal. 2024. V. 60, N 3. Р. 91-109. https://www.dl.begellhouse.com/ru/references/38cb2223012b73f2,0cc841513779c776,54591c6f18601e57.html
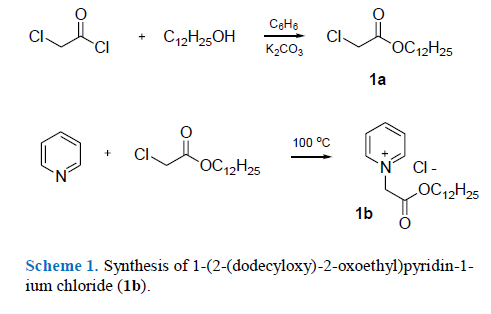
88. Rogalsky S.P., Tarasyuk O.P., Bulko O.V., Lioshyna L.G. Evaluation of growth-promoting effect of 1-(2-(dodecyloxy)-2-oxoethyl) pyridin-1-ium chloride on wheat seedlings. Evaluation of growth-promoting effect of 1-(2-(dodecyloxy)-2-oxoethyl) pyridin-1-ium chloride on wheat seedlings. Ukrainica Bioorganica Acta. 2023. V. 18, N 2. Р. 41-45. DOI: https://doi.org/10.15407/bioorganica2023.02.041
 89. Pilyo S., Kachaeva M., Severin O., Kozachenko O., Zhirnov V., Brovarets V. Design, synthesis, in silico, and in vitro investigatresision of 4-cyano-2-phenyl-1,3-oxazol-5-sulfonamide derivatives. Ukrainica Bioorganica Acta. 2024. V. 19, N 1. P. 33-46. https://bioorganica.com.ua/index.php/journal/article/view/82
89. Pilyo S., Kachaeva M., Severin O., Kozachenko O., Zhirnov V., Brovarets V. Design, synthesis, in silico, and in vitro investigatresision of 4-cyano-2-phenyl-1,3-oxazol-5-sulfonamide derivatives. Ukrainica Bioorganica Acta. 2024. V. 19, N 1. P. 33-46. https://bioorganica.com.ua/index.php/journal/article/view/82
90. Buziashvili А.Yu., Biliavska L.О., Tsygankova V.А., Iutynska G.O., Yemets А.І. Investigation of the influence of avermectin-containing preparations on the resistance of tomato lines to fusarium blight in vitro. Фактори експериментальної еволюції організмів. 2023. Т. 32. С. 74-79. https://doi.org/10.7124/FEEO.v32.1539
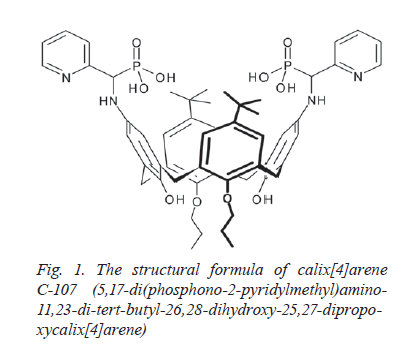
91. Kosterin S.O., Veklich Т.О.,Kalchenko O.І.,Vovk A.I.,Rodik R.V.,Shkrabak О.А. Kinetic regularities and a possible mechanism of ATP non-enzymatic hydrolysis induced by calix[4]arene С-107. Ukrainian Biochemical Journal. 2024. V. 96, N 3. Р. 25-38. doi: https://doi.org/10.15407/ubj96.03.108
 92. Parkhomenko Y.M., Vovk A.I.,Protasova Z.S., Chornyy S.A., Kobzar O.L., Stepanenko S.P., Chekhivska L.I. Molecular structural features that determine the neurotropic activity of thiamine derivatives. Neurophysiology. 2022. V. 54, N 3. Р. 82-93.Published: 12 April 2024. https://doi.org/10.1007/s11062-024-09939-5
92. Parkhomenko Y.M., Vovk A.I.,Protasova Z.S., Chornyy S.A., Kobzar O.L., Stepanenko S.P., Chekhivska L.I. Molecular structural features that determine the neurotropic activity of thiamine derivatives. Neurophysiology. 2022. V. 54, N 3. Р. 82-93.Published: 12 April 2024. https://doi.org/10.1007/s11062-024-09939-5
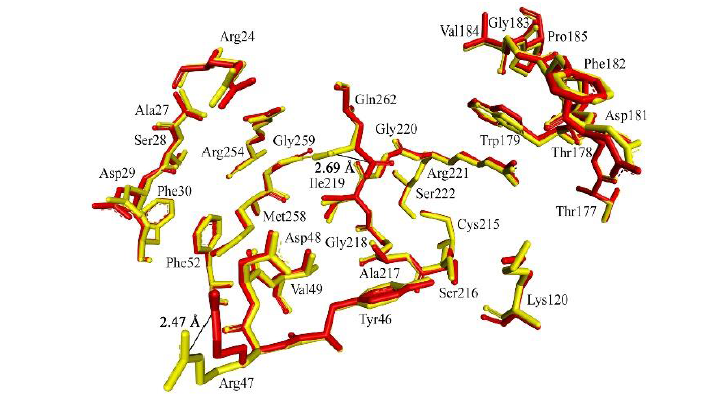
93. Tanchuk V.Y.,Kobzar O.L.,Vovk A.I. Classification of active site conformations of protein tyrosine phosphatase 1B revisited. Ukrainica Bioorganica Acta. 2024. V. 19, N 1. Р. 54-60. https://bioorganica.com.ua/index.php/journal/article/view/84
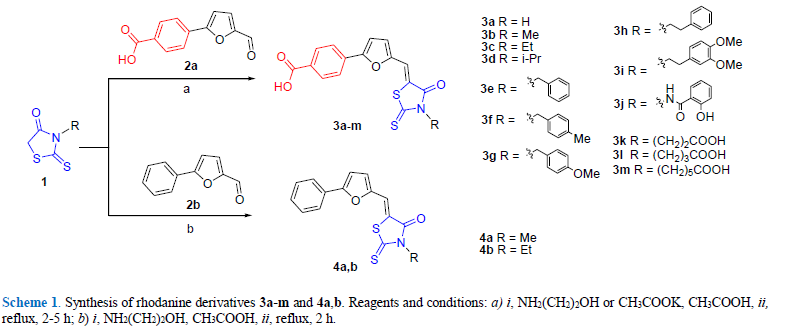
94. Beiko A.V.,Kobzar O.L.,Kachaeva M.V.,Pilyo S.G.,Kozachenko O.P.,Vovk A.I. Rhodanine-based 4-(furan-2-yl)benzoic acids as inhibitors of xanthine oxidase. Ukrainica Bioorganica Acta, 2023, V.18, N 2.Р. 31-40. DOI: https://doi.org/10.15407/bioorganica2023.02.031
95. Beiko, A.V.; Kobzar, O.L.; Kachaeva, M.V.; Pilyo, S.G.; Tanchuk, V.Y.; Vovk, A.I. Inhibition of xanthine oxidase by pyrazolone derivatives bearing a 4-(furan-2-yl)benzoic acid moiety. Journal of Organic and Pharmaceutical Chemistry. 2023. 21, 27-35. DOI: https://doi.org/10.24959/ophcj.23.298726
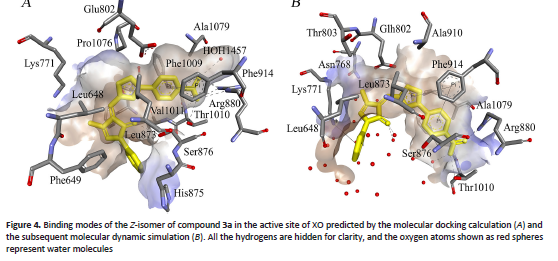
96. Y.S. Kolesnikov, S.V. Kretynin, V.S. Kravets, Y.K. Bukhonskа. Phosphatidic acid formation and signaling in plant cells. Ukr. Biochem. J. 2024. V. 96, N 1. doi: https://doi.org/10.15407/ubj95.06
97. A. Wzorek, J. Han, N.V. Lyutenko, M. Koley, A.E. Sorochinsky, T. Ono, V.A. Soloshonok. Enzymatic approaches for preparation of α-aminophosphonic acids and fluorine-containing β-amino acids. Ukrainica Bioorganica Acta, 2024. V. 19, N 1. P. 21-32. https://bioorganica.com.ua/index.php/journal/article/view/81
98. J. Han, A. Wzorek, G. Dhawan, W. Zhang, A.E. Sorochinsky, T. Ono, V.A. Soloshonok. New drugs appearing on the market in 2023: molecules containing fluorine and fragments of tailor-made amino acids. Ukrainica Bioorganica Acta, 2024. V. 19, N 1. Р. 3-20. https://bioorganica.com.ua/index.php/journal/article/view/79
99. Kretynin S.V., Kolesnikov Y.S., Kravets V.S., Blume Ya.B. Effect of 28-Homobrassinolide on Fatty Acid Metabolism During Germination of Crambe tatarica Under Salinity Stress. ytol. Genet. 2024. V. 58. P. 21-28. https://doi.org/10.3103/S0095452724010043
100. D.Z. Davitadze, S.V. Konovalov. Regularities of epoxidized alkyl oleates ring-opening reactions with alcohols, water and organic acids in the presence of commercial sulfonated resins as catalysts. Каталіз та нафтохімія. 2024. № 35. C. 10-19. https://doi.org/10.15407/kataliz2024.35.072
101. Bodachivska L.Yu. Use of synthesised ultradispersed substances in technological systems. Catalysis and Petrochemistry. 2024. № 35. С. 55-61. https://doi.org/10.15407/kataliz2024.35.107
1. Tsygankova V.A.. Novel Aspects on Chemistry and Biochemistry. Book Publisher International. SCIENCEDOMAIN international Ltd. India.United Kingdom. Vol.1. 2023. 178 p. ISBN 978-81-19217-26-7 (Print), ISBN 978-81-19217-06-9 (eBook). DOI: 10.9734/bpi/nacb/v1

2. Tsygankova V.A., Spivak S.I., Shysha E.N., Pastukhova N.L., Biliavska L.A., Iutynska G.A., Kyrylenko V.M., Yemets A.I., Blume Ya.B.. The role of polycomponent biostimulants in increasing plant resistance to the biotic and abiotic stress factors. Chapter in Monograph: Agricultural Research Updates. Nova Science Publishers, Inc., NY, USA. 2023, Ed. Prathamesh Gorawala and Srushti Mandhatri, 307 p. ISBN 979-8-89113-332-7 https://novapublishers.com/shop/agricultural-research-updates-volume-46/.

3. A.O. Kolodiazhna,O.I. Kolodiazhnyi. Chiral Organophosphorus drugs. Symmetry. 2023. V.15, N. 8. Р.1550. https://doi.org/10.3390/sym15081550
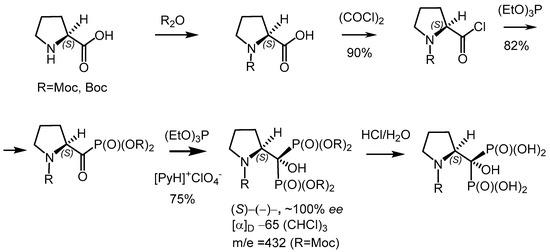
4. D. Kamenskyh, T. Tkachenko, L. Tecer, Y. Sheludko, V. Povazhny, M. Jafarov, V. Yevdokymenko. Influence of ratio of silicon complex and coagulant on silicon dioxide physicochemical characteristics. Applied Nanoscience. 2023. V. 13, N 10. P. 6967-6999. https://doi.org/10.1007/s13204-023-02841-2
5. M. Aksylenko, E. Sheludko, V. Yevdokymenko, O. Haidai, N. Khimach. Biostimulating Effect of Polygalacturonates of Biogenic Metals on Growing Winter Wheat. Cutting Edge Research in Biology. 2023. V. 6, N 11., Ch. 1. P. 1-24, https://doi.org/10.9734/bpi/cerb/v6/5047B

6. M.A. Jafarov, V.O. Yevdokymenko, D.S. Kamenskyh, K.A. Rustamov, Z.A. Jafarov. Mathematical Model Desublimation Conditions. Asian Journal of Chemical Sciences. 2023. V. 13, N 2. P. 1-6. https://doi.org/10.9734/AJOCS/2023/v13i2234
7. M.A. Jafarov, V.O. Yevdokymenko, D.S. Kamenskyh, K.A. Rustamov, Z.A. Jafarov. Mathematical Modeling of Hexafluorsilicate Ammonia Desublimation. Chemical Science International Journal. 2023. V. 32, N 3. P. 62-68. https://doi.org/10.9734/CSJI/2023/v32i3849
8. K. Hutsul, I. Ivanenko, L. Patrylak, O. Pertko, D. Kamenskyh. ZnO/Zeolite composite photocatalyst for dyes degradation. Applied Nanoscience. 2023. Р. 1-9. https://doi.org/10.1007/s13204-023-02950-y
9. M.A. Jafarov, V.O. Yevdokymenko, D.S. Kamenskyh, K.A. Rustamov, Z.A. Jafarov. Mathematical Modeling of Hexafluorsilicate Ammonia Desublimation. Evolutions Mech Eng. 2023. V. 4, N 4. EME.000595.2023. https://doi.org/10.31031/EME.2023.04.000595
10. Vretik L.O., Noskov Yu.V., Chepurna O.M., Ogurtsov N.A., O.A. Nikolaeva, O.A. Marynin, A.I. Ohulchanskyy, A.A. Pud . Dual Stimuli-Responsive Ternary Core-Shell Polystyrene@Pnipam-Pedot Latexes. Particle & Particle Systems Characterization. 2023. Р. 2300096. https://doi.org/10.1002/ppsc.202300096
11. Petrychuk M.V., Oliynyk V.V., Zagorodnii V.V.,Ogurtsov N.A., Pud A.A. PVDF/poly(3-methylthiophene)/MWCNT nanocomposites for EMI shielding in the microwave range. Heliyon. 2023. V. 9, N 12. Р. e23101. https://doi.org/10.1016/j.heliyon.2023.e23101

12. Z.I. Kazantseva, I.A. Koshets, A.V. Mamykin, A.S. Pavluchenko, O.L. Kukla, A.A. Pud, N.A. Ogurtsov, Yu.V. Noskov, R.V. Rodik, S.G. Vyshnevskyy. Detection of the explosive nitroaromatic compound simulants with chemosensory systems based on quartz crystal microbalance and chemiresistive sensor arrays. Semiconductor Physics, Quantum Electronics & Optoelectronics. 2023. V. 26, N.3. Р. 332-342. https://doi.org/10.15407/spqeo26.03.332
13. N. Redon, N. Davydenko, N. Ogurtsov, M. Jamar, Yu. Noskov, A. Pud, J.-L. Wojkiewicz. PPy & P3MT-MWCNT Nanocomposites-Based Sensors for Nerve Gas Detection at ppb Levels. 2023 IEEE SENSORS proceedings, 2023, Vienna, Austria. Р. 1-4. DOI:10.1109/SENSORS56945.2023.10325230
14. I.P. Matushko, Yu.V. Noskov,V.A. Moiseienko,V.Y. Malyshev,L.M. Grishchenko. Electromagnetic Microwave Absorption Performances of PVC/AC Composites. Materials Proceedings. 2023. V. 14, N.1. Р. 15. https://doi.org/10.3390/IOCN2023-14537
15. S. Konovalov, S. Zubenko, L. Patrylak, A. Yakovenko, V. Povazhnyi, K. Burlachenko. On the Peculiarities of Alkaline-Catalyzed Route of Synthesis of Fatty Acid Monoalkyl Esters. International Symposium on Electric Aircraft and Autonomous Systems. Advances in Electric Aviation. 2023/ Р. 275-281. DOI:10.1007/978-3-031-32639-4_35
16. Patrylak L.K., Yakovenko A.V., Nizhnik B.O., Pertko O.P., Povazhnyi V.A., Kamenskyh D.S., Melnychuk O.V.. Natural Zeolites Modified with Silver Nanoparticles as Promising Sorbents with Antibacterial Properties. Nanoelectronics, Nanooptics, Nanochemistry and Nanobiotechnology, and Their Applications. Springer Proceedings in Physics. 2023. Р. 87-98. DOI:10.1007/978-3-031-42708-4_5
17. Kobzar O.L.,Tatarchuk, A.V.,Mrug G.P.,Bondarenko S.P., Demydchuk B.A., Frasinyuk M.S.,Vovk A.I.. Carboxylated chalcones and related flavonoids as inhibitors of xanthine oxidase. Medicinal Chemistry Research. 2023. V. 32, N 8. Р. 1804-1815. https://doi.org/10.1007/s00044-023-03109-8
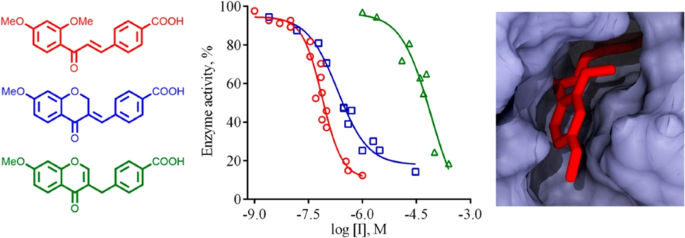
18. Velihina Y.,Gesese R.,Zhirnov V.,Kobzar O.,Bui B.,Pilyo S.,Vovk A.,Shen H.Brovarets V.. Design, synthesis and evaluation of the anti-breast cancer activity of 1,3-oxazolo[4, 5-d] pyrimidine and 1, 3-oxazolo[5, 4-d] pyrimidine derivatives. RSC Medicinal Chemistry. 2023. V. 14, N 4.Р. 692-699. https://doi.org/10.1039/D2MD00377E
19. Kovalishyn V.,Severin O.,Kachaeva M.,Kobzar O.,Keith K.A.,Harden E.A.,Hartline C.B.,James S.H.,Vovk A.,Brovarets V.. In Silico Design and Experimental Validation of Novel Oxazole Derivatives Against Varicella zoster virus. Molecular Biotechnology. 2023. Р. 1-11. https://doi.org/10.1007/s12033-023-00670-w
20. Los O.V.,Sinenko V.O.,Kobzar O.L.,Zhirnov V.V.,Vovk A.I.,Brovarets V.S.. Synthesis and in vitro anticancer potential of new thiazole-containing derivatives of rhodanine. Chemistry of Heterocyclic Compounds. 2023. V. 59, N 6-7. Р. 484-493. https://doi.org/10.1007/s10593-023-03220-z

21. Zhirnov V.,Shablykin O., Chumachenko S.,Kornii Y.,Keith K. A.,Harden E. A.,Hartline C.B.,James S.H.,Kobzar O.,Kovalishyn V.,Vovk A.Brovarets V.. In vitro activity of novel 4-iminohydantoin sulfamide derivatives against human cytomegalovirus. Chemical Papers. 2023. Р. 1-8. https://doi.org/10.1007/s11696-023-03038-1
22. Hodyna D.,Kovalishyn V., Kachaeva M.,Shulha Y.,Klipkov A.,Shaitanova E.,Kobzar O.,Shablykin O.,Metelytsia L.. In Silico, In Vitro and In Vivo Study of Substituted Imidazolidinone Sulfonamides as Antibacterial Agents. Chemistry & Biodiversity. 2023. Р. e202301267. DOI: 10.1002/cbdv.202301267
23. Derevyanchuk M.Kretynin S., Bukhonska Y., Pokotylo I., Khripach V., Ruelland E., Filepova R., Dobrev P.I., Martinec, J., Kravets V.. Influence of Exogenous 24-Epicasterone on the Hormonal Status of Soybean. Plants. 2023. V.12. N 20. Р. 3586. https://doi.org/10.3390/plants12203586
24. Kretynin S.V., Kolesnikov Y.S.. The role of calcium in implementation of the effect of brassinosteroids during the induction of oxidative stress in tobacco. Cytology and Genetics. 2023. V. 57. Р. 312-319. https://doi.org/10.3103/S0095452723040072
25. Rogalsky S., Tarasyuk O., Vashchuk A., Dzhuzha O., Cherniavska T., Makhno S.. Thermophysical properties and ionic conductivity of new imidazolium based protic ionic liquids. Journal of Molecular Liquids. 2023. V. 382. Р. 121942. https://doi.org/10.1016/j.molliq.2023.121942
26. Lishchuk P., Vashchuk A., Rogalsky S., Chepela L., Borovyi M., Lacroix D., Isaiev M.. Thermal transport properties of porous silicon filled by ionic liquid nanocomposite system. Scientific Reports. 2023. V.13. Р. 5889. DOI:10.1038/s41598-023-32834-8
27. Rogalsky S., Tarasyuk O., Babkina N., Makhno S., Pertko O., Povazhnyi V., Cherniavska T., Fatyeyeva K.. Fabrication of new proton conducting membrane for fuel cell applications based on porous polyimide Matrimid® and hydrophobic protic ionic liquid. Journal of Applied Polymer Science. 2023. V. 140, N 15. Р. e53731. https://doi.org/10.1002/app.53731
28. Talaniuk V., Godzierz M., Vashchuk A., Iurhenko M., Chaber P., Sikorska W., Kobyliukh A., Demchenko V., Rogalsky S., Szeluga U., Adamus G.. Development of Polyhydroxybutyrate – Based Packaging Films and Methods to Their Ultrasonic. Materials. 2023. V.16, N 20. Р. 6617. https://doi.org/10.3390/ma16206617
29. Rogalsky S., Hodyna D., Semenyuta I., Frasinyuk M., Tarasyuk O., Riabov S., Kobrina L., Tetko I., Metelytsia L.. Antibacterial activity of 1-dodecylpyridinium tetrafluoroborate and its inclusion complex with sulfobutyl ether-b-cyclodextrin against MDR Acinetobacter baumannii strains. Innovative Biosystems and Bioengineering. 2023. V.7, N 4.Р. 25-35. https://doi.org/10.20535/ibb.2023.7.4.288529
30. Vortman M.Ya., Berezhnytska O.S., Aksenovska O.A., Kobylinskyi S.M., Kobrina L.V., Lemeshko V.N., Shevchenko V.V.. Guanidinium-containing oligoether as a complexing agent of transition metal ions. Functional Materials. 2023. V.30, N 1. Р. 120-127. https://doi.org/10.15407/fm30.01.120
31. Patrylak L.,Konovalov S.,Yakovenko A.,Pertko O.,Povazhnyi V.. Polycationic Nanostructured Faujasite Zeolite Catalysts for Glucose Transformation into 5-Hydroxymethylfurfural. Applied Nanoscience. 2023,V. 13. Р. 5743–5754. https://doi.org/10.1007/s13204-023-02820-7
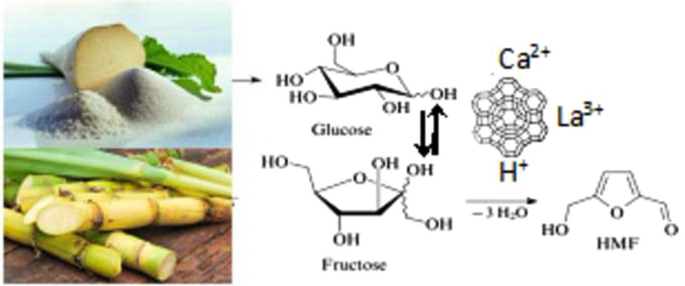
32. Shvets O.V.,Kurmach M.M., Yaremov P.S., Voloshyna Yu.G., Shcherban N.D.. Zeolite nanocomposites with variable acid and basic properties: effective catalysts for fine chemical synthesis and industrial reaction. Applied Nanoscience. 2023. https://doi.org/10.1007/s13204-023-02955-7
33. I. Semenyuta, D. Hodyna, V. Kovalishyn, B. Demydchuk, M. Kachaeva,S. Pilyo, V. Brovarets, L. Metelytsia. Development and application of in silico models to design new antibacterial5-amino-4-cyano-1,3-oxazoles against colistin-resistant E. coli strains. Artificial Intelligence Chemistry. 2023. V. 1, N 2. Р. 100024. https://doi.org/10.1016/j.aichem.2023.100024
34. D. Hodyna, V. Kovalishyn, Y. Romanenko, I. Semenyuta,V. Blagodatny, M. Kachaeva, O. Brazhko L. Metelytsia. Quinoline Hydrazone Derivatives as New Antibacterialsagainst Multidrug Resistant Strains. Chem. Biodiversity 2023. V. 20, N 10. Р. e202300839. doi.org/10.1002/cbdv.2023008

35. D. Hodyna,V. Kovalishyn,I. Semenyuta,M. Kachaeva,Y. Shulha,M. Bugera, V. Blagodatny, O. Shablykin,L. Metelytsia. Design and Biological Evaluation of 4-Iminohydantoin Sulfamides as New Anti-Acinetobacter baumannii Agents. Biointerface Res. Appl. Chem. 2023. V. 13, N 6. P. 511-524.
36. G. Mrug,D. Hodyna,L. Metelytsia,V. Kovalishyn,O. Trokhimenko, S. Bondarenko,K. Kondratyuk,A. Kozitskiy, M. Frasinyuk. Structure-Activity Relationship Prediction-Based Synthesis and Cytotoxicity Evaluation against the HEp-2 Laryngeal Carcinoma Cell of Isoflavone–Cytisine Mannich Bases. Chemistry & Biodiversity. 2023. V. 20, N 8. Р. e202300560. https://doi.org/10.1002/cbdv.202300560

37. Pilyo S.G., Demydchuk B.A., Moskvina V.S., Shablykina O.V., Brovarets V.S.. A combinatorial library of substituted 3-sulfonyl-2-imino-1,2-dihydro-5H-dipyrido[1,2-a:2′,3′-d]pyrimidin-5-ones and their anticancer activities. Biopolym. Cell. 2022. V. 38, N 4. Р. 242-256. http://dx.doi.org/10.7124/bc.000A7B
38. Nizhenkovska I.V., Matskevych K.V., Golovchenko O.I., Golovchenko O.V., Kustovska A.D., Van M.. New prospective phosphodiesterase inhibitors: phosphorylated oxazole derivatives in treatment of hypertension. Adv. Pharm. Bull. 2023. V.13, N 2. С. 399-407. https://doi.org/10.34172/apb.2023.044
39. Zyabrev V., Demydchuk B., Zhirnov V., Brovarets V.. Synthesis, characterization, and in vitro anticancer evaluation of 2-aryl-4-arylsulfonyl-5-RS-1,3-oxazoles. Biointerface Res. Appl. Chem. 2023. V.13, N 3. P. 300. DOI: 10.33263/BRIAC133.300
40. Kovalishyn V., Severin O., Kachaeva M., Semenyuta I., Keith K.A., Harden E.A., Hartline C.B., James S.H., Metelytsia L., Brovarets V.. Design and experimental validation of the oxazole and thiazole derivatives as potential antivirals against of human cytomegalovirus. SAR and QSAR in Environmental Res. 2023. V.34, N 7. P. 523-541. DOI: 10.1080/1062936X.2023.2232992
41. Bondar D., Bragina O., Lee Ji Y., Semenyuta I., Jӓrving I., Brovarets V., Wipf P., Bahar I., Karpichev Y.. Hydroxamic Acids as PARP-1 Inhibitors: Molecular Design and Anticancer Activity of Novel Phenanthridinones. Helv. Chim. Acta. 2023. https://doi.org/10.1002/hlca.202300133
42. Zyabrev V., Pilyo S., Demydchuk B., Kachaeva M., Semenyuta I., Zhirnov V., Velihina Ye., Brovarets V.. Synthesis, characterization and in vitro anticancer evaluation of 5-sulfinyl(sulfonyl)-4-arylsulfonyl substituted 1,3-thiazoles. Chem. Med. Chem. 2023. V.18, N 14. Р. E202300161. https://doi.org/10.33263/BRIAC133.300
43. Shablykin O.V., Merzhievskyi D.O., Brovarets V.S., Shishkina S.V.. New oxazole to oxazole recyclization. Chem. Het. Compd. 2023. V. 59, N 6. P. 521-524. https://doi.org/10.1007/s10593-023-03226-7
44. Konovalenko A.S., Shablykin O.V., Shablykina O.V., Moskvina V.S., Shishkina S.V., Kozytskyi A.V., Brovarets V.S.. Distinctive features of 3-acetyl- and 3-benzoylisocoumarins’ interaction with active primery amines. Chem. Select. 2023. V.8, N 37. Р. e202301380. https://doi.org/10.1002/slct.202301380.

45. Golovchenko O.V., Brusnakov M.V., Shabelko Yu.O., Brovarets V.S., Vydzhak R.M., Bahrieieva O.S., Potikha L.M., Shishkina S.V.. Synthesis and properties of methanesulfonyl derivatives of diethyl esters of 5-(hydroxyalkylamino)-1,3-oxazol-4-yl-phosphonic acids. Phosphorus, Sulfur, and Silicon and the Related Elements. 2023. DOI: 10.1080/10426507.2023.2251639

46. Shaydyuk Ye.O., Bashmacova N.V., Klishevich G.V., Dmytruk A.M., Kachkovsky O.D., Kuziv Ya.B., Dubey I.Ya., Befield K.D., Bondar M.V.. Nature of linear spectra; properties and fast relaxations in the excited states and two-photon absorption efficiency of 3-thiazolyl and 3-phenylthiazolyl coumarin derivatives. ACS Publications. 2023. V.18, N 12. P. 11564-11573. https://doi.org/10.1021/acsomega.3c00654

47. Brusnakov M.Yu., Golovchenko O.V., Potikha L.M., Brovarets V.S.. Condenced azole-based organophosphorus heterocycles. Chem. Het. Compd. 2023. V.59, N 4/5. P. 217-236. Doi:10.1007/s10593-023-03184-0
48. Konovalenko A., Shablykin O., Shablykina O., Kozytskyi A., Brovares V. Convenient and versatile method of 8-amino-6-(2-R-thiazol-4-yl)1,7-naphthyridines. Curr. Chem. Lett. 2024. V.13, N 1. P. 163-172. Doi: 10.5267/j.ccl.2023.7.004
49. Nallaparaju J.V., Nikonovich T., Jarg T., Merzhyievskyi D., Aav R., Kananovich D.G.. Mechanochemistry – amended barbier reaction as an expedient alternative to Grignar synthesis. Angew. Chem. Int. Edd. 2023. E202305775. https://doi.org/10.1002/anie.202305775

50. Dibchak D., Snisarenko M., Mishuk A., Shablykin O., Bortnichuk L., Klymenko-Ulianov O., Kheylik Yu., Sadkova I., Rzepa H.S., Mykhailiuk P.K.. General synthesis of 3‐azabicyclo [3.1.1]heptanes and evaluation of their properties as saturated isosteres. Angewandte Chemie. 2023. V.135, N 39. Р. e202304246. https://doi.org/10.1002/ange.202304246

51. Kirichok A.,Tkachuk H., Kozyriev Ye., Shablykin O., Datsenko O., Granat D., Yegorova T., Bas Yu., Semirenko V., Pishel I., Kubyshkin V., Lesyk D., Klymenko-Ulianov O., Mykhailiuk P.K.. 1‐Azaspiro[3.3]heptane as a bioisostere of piperidine. Angewandte Chemie. 2023. e202311583. https://doi.org/10.1002/ange.202311583

52. Krasylov I.V., Moskvina V.S., Khilya V.P.. Unexpected but prominent imines formation in Beckmann rearrangement of (spiro)pyranocoumarin oximes. Tetrahedron Lett. 2023. V. 129. P. 154747. https://doi.org/10.1016/j.tetlet.2023.154747

53. Chernykh A.V., Kudryk O.V., Olifir O.S., Dobrydnev A.V., Rusanov E., Moskvina V.S., Volochnyuk D.M., Grygorenko O.O.. Expanding the chemical space of 1,2-difunctionalized cyclobutanes. J. Org. Chem. 2023. V. 88, N 5. P. 3109-3131. https://doi.org/10.1021/acs.joc.2c02892

54. Chen X., Lv X., Gao L., Liu J., Wang W., Guo L., Frasinyuk M. S., Zhang W., Watt D. S., Liu C., Liu X.. Chalcone derivative CX258 suppresses colorectal cancer via inhibiting the TOP2A/Wnt/β-Catenin signaling. Cells. 2023. V.12, N 7. P. 1066. https://doi.org/10.3390/cells12071066
55. Myshko A., Mrug G., Kondratyuk K., Demydchuk B., Bondarenko S., Frasinyuk M.. An expedient synthesis of functionalized pyrazole-based aurone analogs.. Chemistry Select. 2023. V.8, N 20. Р. e202300257.

56. Myshko N.V., Mrug G.P., Kondratyuk K.M., Bondarenko S.P., Frasinyuk M.S.. Coumarin-based homoisoflavonoids as precursors in the synthesis of 8-heteroarylmethylcoumarins. Chem. Heterocycl. Compd. 2023. V. 59, N 6/7. P. 456-464. https://doi.org/10.1007/s10593-023-03216-9
57. Levterov V., Panasyuk Ya., Sahun K., Stashkevich O., Badlo V., Shablykin O., Sadkova I., Bortnichuk L., Klymenko-Ulianov O., Holota Yu., Lachmann L., Borysko P., Horbatok K., Bodenchuk I., Bas Yu., Dudenko D., Mykhailiuk P.K.. 2-Oxabicyclo [2.2.2]octane as a new bioisostere of the phenyl ring. Nature Commun. 2023. V.14, N 1. P. 5608. https://doi.org/10.1038/s41467-023-41298-3
58. Goulden T., Bodachivskyi Iu., Padula M.P., Williams D.B.G.. Concentrated ionic liquids for proteomics: Caveat emptor. International Journal of Biological Macromolecules. 2023. V. 253, № 7. Р. 127438. https://doi.org/10.1016/j.ijbiomac.2023.127438

59. Kornii Yu., Shablykin O., Tarasiuk T., Stepaniuk O., Matvienko V., Aloshyn D., Zahorodniuk N., Sadkova I.V., Mykhailiuk P.K.. Fluorinatedaliphatic diazirines: preparation, characterization, and model photolabeling studies. J. Org. Chem. 2023. V. 88, N 1. P. 1-17. Doi:10.1021/acs.joc.2c02262

60. Malets Ye. S., Vashchenko B. V., Moskvina V.S., Golovchenko O.V., Brovarets V.S., Grygorenko O.O.. Parent 5(7)-azachromones and their partially hydrogenated derivatives: synthesis and physiochemical properties. Chem. Heterocycl. Comp. 2023. V.59, N 6/7. P. 494-499. https://doi.org/10.1007/s10593-023-03221-y
61. Kukushkina K.V., Moskvina V.S., Shablykina O.V., Khilya V.P.. Expanding the isoflavone, pyrazole, and oxazole chemical space through 2′-carboxamido-2-hydroxy-deoxybenzoin precursors.. Chem. Heterocycl. Comp. 2023. V.59, N 6/7. P. 479-483. https://doi.org/10.1007/s10593-023-03219-6
62. Lyutenko N.V., Sorochinsky A.E., Soloshonok V.A.. Asymmetric synthesis of pyroglutamic acids via Ni(II) complex methodology. Chem. Heterocycl. Comp. 2023. Р. 332-340. https://doi.org/10.1007/s10593-023-03203-0

63. Shablykin O.V., Brovarets V.S., Shablykina O.V.. Recyclization of 5-aminooxazoles as a route to new functionalized heterocycles (developments of V.P. Kukhar institute of bioorganic chemistry and petrochemistry of the NAS of Ukraine). Chem. Record. 2023. e202300264. https://doi.org/10.1002/tcr.202300264
64. Gaponov A.M., Pavlenko O.L., Dmytrenko O.P., Kulish M.P., Ryzhkova A.S., Lesiuk A.I., Obernikhina N.V., Łuszczyńska B., Kachkovsky O.D.. Molecular heteroassociation in films of thiochrome and tryptophan. Mol. Cryst. Liquid Cryst. 2023. https://doi.org/10.1080/15421406.2023.2257515
65. Tsygankova V.A., Andrusevich Ya.V., Pilyo S.G., Brovarets V.S.. Effect of plant growth regulators and fertilizers of the vegetative growth of sunflowers (Helianthus annuus L). The scientific heritage. 2023.N 116. P. 3-9. https://zenodo.org/badge/DOI/10.5281/zenodo.8129039.svg
66. Tsygankova V.A., Andreev A.M., Andrusevich Ya.V., Pilyo S.G., Klyuchko S.V., Brovarets V.S.. Use of synthetic plant growth regulators in combination with fertilizers to improve wheat growth. Int J Med Biotechnol Genetics. 2023. S1:02:002:9-14. URL: http://scidoc.org/IJMBGS1V2.php
67. Tsygankova V.A., Voloshchuk I.V., Kopich V.M., Pilyo S.G., Klyuchko S.V., Brovarets V.S.. Studying the effect of plant growth regulators Ivin, Methyur and Kamethur on growth and productivity of sunflower. J. Advanc. Agricult. 2023. V.14. P. 17-24. DOI: https://doi.org/10.24297/jaa.v14i.9453
68. Tsygankova V.A., Andrusevich Ya.V., Kopich V.M., Voloshchuk I.V., Pilyo S.G., Klyuchko S.V., Brovarets V.S.. Application of pyrimidine and pyridine derivatives for regulation of chickpea (Cicer arietinum L.) growth. Int. J. Innovat. Sci. Res. Techn. (IJISRT). 2023. V.8, N 6. P. 19-28. DOI: https://doi.org/10.5281/zenodo.8020671
69. Tsygankova V.A., Kopich V.M., Voloshchuk I.V., Pilyo S.G., Klyuchko S.V., Brovarets V.S.. New growth regulators of barley based on pyrimidine and pyridine derivatives. Sciences of Europe. 2023. N. 124. P. 13-23. https://doi.org/10.5281/zenodo.8327852.
70. Tsygankova V.A., Andrusevich Ya.V., Kopich V.M., Voloshchuk I.V., Bondarenko O.M., Pilyo S.G., Klyuchko S.V., Brovarets V.S.. Effect of pyrimidine and pyridine derivatives on the growth and photosynthesis of pea microgreens. Int. J. Med. Biotechnol. Genetics. 2023. S1:02:003:15-22. https://UBAscidoc.org/IJMBGS1V2.php
71. Tsygankova V.A., Andreev A.M., Andrusevich Ya.V., Kopich V.M., Klyuchko S.V., Pilyo S.G., Brovarets V.S.. Use of Ivin, Methyur, Kamethur and microfertilizers to improve the growth of oilseed flax (Linum usitatissimum L.). Annali d’Italia. 2023. N. 48. P. 3-10. https://doi.org/10.5281/zenodo.10034698.
72. Tsygankova V.A., Andreev A.M., Andrusevich Ya.V., Kopich V.M., Pilyo S.G., Klyuchko S.V., Brovarets V.S.. Synergistic effect of synthetic plant growth regulators and microfertilizers on the growth of canola (Brassica napus L.). Danish Scient. J. (DSJ). 2023. V.1, N. 77. P. 8-12. https://doi.org/10.5281/zenodo.10053315
73. Tsygankova V.A., Voloshchuk I.V., Andrusevich Ya.V., Kopich V.M., Oliynyk O.O., Stefanovska T.R., Pidlisnyuk V., Pilyo S.G., Klyuchko S.V., Brovarets V.S.. Use of synthetic plant growth regulators in agriculture and biotechnology.. Polish J. Sci. 2023. V. 1, N. 68. P. 12-17. https://doi.org/10.5281/zenodo.10131991.
74. Severin O.O., Kachaeva M.V., Pilyo S.G., Kovalishyn V.V., Keith K.A., Harden E.A., Hartline C.B., James S.H., Zhirnov V.V., Brovarets V.S.. Synthesis, characterization and study of anti-HPV activity and cell cytotoxicity of novel 1,3-oxazole-4-carbonitrile and 4-sulfonylamide-5-phenyl-1,3-thiazole derivatives in vitro. Letters in Applied Nanobioscience. 2023. https://doi.org/10.33263/LIANBS132.089
75. Denisenko A., Garbuz P., Makovetska Ye., Shablykin O., Lesyk D., Al-Maali G., Korzh R., Sadkova I., Mykhailiuk P.. 1,2-Disubstituted bicyclo[2.1.1]hexanes as bioisosteres of the ortho-substituted benzene. Chem. Sci. 2023. 10.1039/D3SC05121H. https://doi.org/10.1039/D3SC05121H
76. E. Shaitanova, V. Matoušek, T. Herentin, M. Adamec, R. Matyáš, B. Klepetářová, P. Beier. Synthesis and Cycloaddition Reactions of 1-Azido-1,1,2,2-tetrafluoroethane. J. Org. Chem. 2023. V. 88, N 21. Р. 14969-14977. https://doi.org/10.1021/acs.joc.3c01346

77. J. He, Z. Li, G. Dhawan, W. Zhang, A. E. Sorochinsky, G. Butler, V. A. Soloshonok, J. Han. Fluorine-containing drugs approved by the FDA in 2021. Chinese Chemical Letters. 2023. V. 34. Р. 107578. https://doi.org/10.1016/j.cclet.2022.06.001

78. N.V. Lyutenko, A.E. Sorochinsky, V.A. Soloshonok. Asymmetric synthesis of pyroglutamic acids via Ni(II)-complex methodology. Chemistry of Heterocyclic Compounds. 2023. V. 59. Р. 332-340. https://doi.org/10.1007/s10593-023-03203-0

79. Romanenko V.D.. Synthetic strategies toward and around the CF3S(O) structural motif. Current Organic Chemistry. 2023. V. 27. Р.411-434. https://doi.org/10.2174/1385272827666230517114921

80. Holovach S.,Melnykov K.P.,Poroshyn I.,Iminov R.T.,Dudenko D.,Kondratov I.S.,Levin M.,Grygorenko O.O.. C−C Coupling through Nitrogen Deletion: Application to Library Synthesis. Chemistry A European Journal. 2023. V. 29, N 4.Р. e202203470. https://doi.org/10.1002/chem.202203470

81. Fink E. A., Bardine C.,Gahbauer S., Singh I.Detomasi T. C.,White K., Gu S.,Wan X., Chen J.,Ary B., Glenn I.,O’Connell J.,O’Donnell H.,Fajtov P., Lyu J.,Vigneron S.,Young N.J.,Kondratov I.S.,Alisoltani A.,Simons L.M.,Lorenzo-Redondo R.,Ozer E. A.,Hultquist J.F.,O’Donoghue A. J.,Moroz Y.S.,Taunton J.,Renslo A.R.,Irwin J.J.,García-Sastre A.,Shoichet B.K.,Craik C.S.. Large library docking for novel SARS‐CoV‐2 main protease non‐covalent and covalent inhibitors. Protein Science. 2023. V. 32. Р. e4712. https://doi.org/10.1002/pro.4712
82. Gahbauer S.,DeLeon C.,Braz J.M.,Craik V.,Kang H.J.,Wan X.,Huang X.-P.,Billesbølle C.B.,Liu Y.,Che T.,Deshpande I.,Jewell M.,Fink E.A.,Kondratov I.S.,Moroz Y.S.,Irwin J.J.,Basbaum A.I.,Roth B.L.,Shoichet B.K.. Docking for EP4R antagonists active againstinflammatory pain. Nature Communications. 2023. V. 14, N 1. Р. 8067. https://doi.org/10.1038/s41467-023-43506-6
83. Rud A.D., Kornienko N.E., Polunkin I.V., Boguslavskii L.Z., Vinnichenko D.V., Kirian I.M., Kolomys O.F., Kuskova N.I.. Structure of carbon nanospheres modified with oxygen-containing groups and halogens. Applied Nanoscience. 2023. № 10. Р. 6929-6937. DOI: https://doi.org/10.1007/s13204-023-02817-2
1. T.V. Tkachenko, D.S. Kamenskyh, Y.V. Sheludko, V.O. Yevdokymenko . Structural and morphological features of nanoсellulose from soybean straw. Nanoobjects & Nanostructuring. Volume I / Edited by Lidiya M. Boichyshyn and Oleksandr. V. Reshetnyak. ‒ Mississauga, Ontario: Nova Printing Inc. 2022. Р. 145-160.
2. Bondar M.V., Faryadras S., Munera N., Chang H.-T., Uddin M., Beldield K.D., Kachkovsky O.D., Van Stryland E.W., Hagan D.J.. New two-photon absorbing Squaraine derivative with efficient near-infrared fluorescence, superluminescence, and high photostability. J. Phys. Chem. B. 2022. V.126, N 21. P. 3897-3907. https://doi.org/10.1021/acs.jpcb.2c01288

3. V.A. Tsygankova, Ya.V. Andrusevich, O.I. Shtompel, R.M. Solomyanny, A.O. Hurenko, M.S. Frasinyuk, G.P. Mrug, O.V. Shablykin, S.G. Pilyo, A.M. Kornienko, V.S. Brovarets. New Auxin and Cytokinin Related Compounds Based on Synthetic Low Molecular Weight Heterocycles. Auxins, Cytokinins and Gibberellins Signaling in Plants. Signaling and Communication in Plants. Aftab, T. (Eds). Springer Nature. Switzerland AG. 2022. 377 p. Pp. 353-377. http://dx.doi.org/10.1007/978-3-031-05427-3_16
4. S. Konovalov,S. Zubenko,L. Patrylak, A. Yakovenko, V. Povazhnyi, K. Burlachenko. Revisiting the Synthesis of Fatty Acid Alkyl Esters of Lower Monohydric Alcohols by Homogeneous Base-Catalyzed Transesterification of Vegetable Oils. Chemmotological Aspects of Sustainable Development of Transport. Sustainable Aviation. 2022, Springer, Cham.Р. 49-80. https://doi.org/10.1007/978-3-031-06577-4_4
5. D. Hodyna, V. Kovalishyn, I. Semenyuta, S. Rogalsky, O. Trokhimenko, A. Gryniukova, L. Metelytsia. Ester-Functionalized Imidazolium- and Pyridinium-Based Ionic Liquids: Design, Synthesis and Cytotoxicity Evaluation. Biointerface Research in Applied Chemistry. 2022. V. 12, N 3. P. 2905-2957. https://doi.org/10.33263/BRIAC123.29052957
6. L.O. Metelytsia,D.M. Hodyna, I.V. Semenyuta,V.V. Kovalishyn,S.P. RogalskyY.K. Derevianko, V.S. Brovarets,I.V. Tetko. Theoretical and Experimental Studies of Phosphonium Ionic Liquids as Potential Antibacterials of MDR Acinetobacter baumannii. Journal of Antibiotics. 2022. V. 11. P. 491. https://doi.org/10.3390/antibiotics11040491.
7. Vydzhak R.N., Panchishin S.Y.,Kachaeva M.V.,Pilyo S.G., Moskvina V.S., Shablykina O.V., Kozytskiy A.V.,Brovarets V.S. Rapid synthetic approaches to libraries of diversified 1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones and 3-(2-hydroxyphenyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-ones. Molecular diversity. 2022. Vol. 26, N 2. Р. 1115-1128. https://doi.org/10.1007/s11030-021-10234-2
8. O.V. Moshynets,T.P. Baranovskyi,O.S. Iungin, N.P. Kysil, L.O. Metelytsia,I. Pokholenko, V.V. Potochilova,G. Potters, K.L. Rudnieva,S.Y. Rymar, I.V. Semenyuta, A.J. Spiers, O.P. Tarasyuk,S.P. Rogalsky eDNA inactivation and biofilm inhibition by the polymeric biocide polyhexamethylene guanidine hydrochloride (PHMG-Cl). International Journal of Molecular Sciences. 2022. V. 23. Р. 731. https://doi.org/10.3390/ijms23020731
9. Obernikhina N.V.,Kachaeva M.V., Kachkovsky O.D.,Brovarets V.S. In silico Study of Conjugated Nitrogen Heterocycles Affinity in their Biological Complexes. Chemistry of Heterocyclic Compounds 2022. V. 58, N 8. Р. 412-420. DOI 10.1007/s10593-022-03107-5
10. D.S. Kamenskyh, T.V. Tkachenko, V.A. Yevdokymenko, Y.V. Sheludko, M.M. Filonenko, V.A. Povazhny, M.M. Baran, O.V. Pavluik, V.I. Kashkovsky Synthesis, characterization and optimization of the aluminum–nickel–molybdenum catalyst for hydrogenation Applied Nanoscience. 2022. P. 1-13. https://doi.org/10.1007/s13204-022-02644-x
11. T.V. Tkachenko, M.M. Baran, V.O. Yevdokymenko, D.S. Kamenskyh, V.I. Kashkovsky Optimization of Ether Production by Proton Current Materials Today: Proceedings. 2022. V. 62, N 15. P. 7643-7649. https://doi.org/10.1016/j.matpr.2022.02.004
12. L.K. Patrylak, S.V. Konovalov, A.V. Yakovenko, O.P. Pertko, V.A. Povazhnyi, Yu.G. Voloshyna, O.V. Melnychuk, M.M. Filonenko Micro–mesoporous kaolin-based zeolites as catalysts for glucose transformation into 5-hydroxymethylfurfural Applied Nanoscience. 2022. P.1-14. https://doi.org/10.1007/s13204-022-02620-5
13. L. Patrylak, S. Zubenko, S. Konovalov, A. Yakovenko, V. Povazhnyi, O. Pertko, Y. Voloshyna, O. Melnychuk Mykhailo Filonenko Іsomerization of limonene on zeolite-containing catalysts based on Кaolin Chemistry Journal of Moldova. 2022. P. 1857-1727. http://dx.doi.org/10.19261/cjm.2022.980
14. V. Pidlisnyuk, A. Mamirova, R.A. Newton, T. Stefanovska, O. Zhukov, V. Tsygankova, P. Shapoval The role of plant growth regulators in Miscanthus × giganteus utilisation on soils contaminated with trace elements Agronomy. 2022. V. 12, N 12. Р. 2999. https://doi.org/10.3390/agronomy12122999
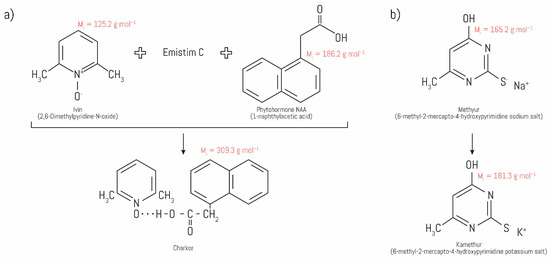
15. Vasetska O., Zhminko P., Prodanchuk M., Galkin A., Tsygankova V. Perspective for using2,6-dimethylpyridine-N-oxide to reduce the toxic effect of xenobiotics in mammals. J. Adv. Pharm. Educ. Res. 2022. V. 12, N 1. P. 21 – 29. https://doi.org/10.51847/TXCxI0PsO1
16. Tsygankova V.A., Voloshchuk I.V., Klyuchko S.V., Pilyo S.G., Brovarets V.S., Kovalenko O.A. The effect of pyrimidine and pyridine derivatives on the growth and productivity of sorghum International Journal of Botany Studies. 2022. V. 7, N 5. P. 19-31. https://www.botanyjournals.com/archives/2022/vol7/issue5/7-4-28
17. Tsygankova V.A., Voloshchuk I.V., Andrusevich Ya.V., Kopich V.M., Pilyo S.G., Klyuchko S.V., Kachaeva M.V., Brovarets V.S. Pyrimidine derivatives as analogues of plant hormones for intensification of wheat growth during the vegetation period Journal of Advances in Biology. 2022. V. 15. P. 1-10. DOI: https://doi.org/10.24297/jab.v15i.9237
18. Tsygankova V.A., Oliynyk O.O., Kvasko O.Yu., Pilyo S.G., Klyuchko S.V., Brovarets V.S. Effect of Plant Growth Regulators Ivin, Methyur and Kamethur on the Organogenesis of Miniature Rose (Rosa mini L.) in Vitro Int. J. Med. Biotechnol. Genetics. 2022. S1: V. 2, N 1. P.1-8. https://scidoc.org/IJMBG-2379-1020-S1-02-001.php
19. Y. Kolesnikov, S. Kretynin, Y. Bukhonska, I. Pokotylo, E. Ruelland, J. Martinec, V. Kravets Phosphatidic Acid in Plant Hormonal Signaling: From Target Proteins to Membrane Conformations Int. J. Mol. Sci. 2022, V. 23, N 6. Р. 3227; https://doi.org/10.3390/ijms23063227
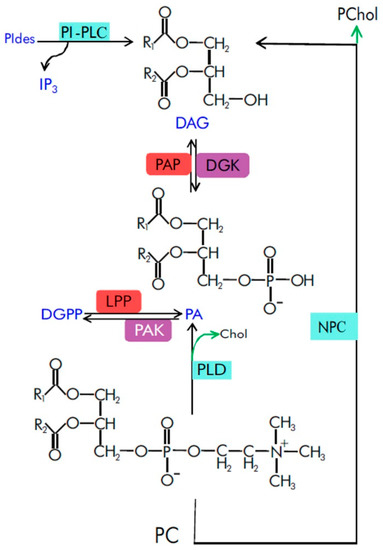
20. Kobzar O.,Shulha Yu.,Buldenko V.,Cherenok S.,Silenko O.,Kalchenko V.,Vovk A. Inhibition of glutathione S-transferases by photoactive calix[4]arene α-ketophosphonic acids. Bioorganic and Medicinal Chemistry Letters. 2022. V. 77. Р. 129019. https://doi.org/10.1016/j.bmcl.2022.129019

21. Silenko O.,Cherenok S.,Shulha Yu.,Kobzar O.,Rusanov E.,Karpichev E.,Vovk A.Kalchenko V. Thiacalix [4] arene phosphoric acids. Synthesis, structure, and inhibition of glutathione S-transferases. Phosphorus, Sulfur, and Silicon and the Related Elements. 2022. V.197 (5-6). Р. 538-541. https://doi.org/10.1080/10426507.2021.2011877

22. Velihina Y.,Pilʹo S.,Kobzar O.,Zaliavska O.,Prichard M. N.,James S. H.Keith K.,Hartline C.,Zhirnov V.,Vovk A.,Brovarets V. Synthesis of some oxazolo [4, 5-d] pyrimidine derivatives and evaluation of their antiviral activity and cytotoxicity. Arkivok. 2022. Р. 108-117.
![Scheme 2. Synthesis of oxazolo[4,5-d]pyrimidines 10-15. Reagents and conditions: (e) piperazine or 1,4-diazepane, dioxane, reflux, 6h; (f) corresponding RSO2Cl, Et3N, dioxane, 105 -110 °C, 6h.](https://www.researchgate.net/profile/Oleksandr-Kobzar-3/publication/358385997/figure/fig1/AS:11431281103572106@1669731526236/Scheme-2-Synthesis-of-oxazolo4-5-dpyrimidines-10-15-Reagents-and-conditions-e.png)
23. Obernikhina N.V., Kobzar O.L.,Kachaeva M.V., Kachkovsky O.D. , Brovarets V.S. In silico and in vitro estimation of structure and biological affinity of 1, 3-oxazoles: fragment-to-fragment approach. Curr. Comput.-Aided Drug Des. 2022. V. 18. P. 95-109. https://doi.org/10.2174/1573409918666220404100022

24. Ivanenko I.,Ruda A.,Povazhnyi V. Cobalt-nitrogen-doped activated carbons for hydrogen generation. Materials Today: Proceedings.2022. V. 62 ( P15). Р. 7691-7697. https://doi.org/10.1016/j.matpr.2022.03.170
25. Yu.G. Voloshyna,O.P. Pertko,V.A. Povazhnyi,L.K. Patrylak Peculiarities of products’ distribution in n-hexane hydroisomerization on modified mordenite-containing rock Appl. Nanosci. 2022. https://doi.org/10.1007/s13204-022-02632-1
26. Yu. Kholodko, A. Bondarieva, V. Tobilko, V. Pavlenko, O. Melnychuk, V. Glukhovskyi. Synthesis and characterization of kaolinite-based granular adsorbents for the removal of Cu(II), Cd(II), Co(II), Zn(II), and Cr(VI) from contaminated water. Eastern-European Journal of Enterprise Technologies.2022. V. 4 N. 10. Р. 118. https://doi.org/10.15587/1729-4061.2022.262994
27. Rogalsky S., Tarasyuk O., Vashchuk A., Davydenko V., Dzhuzha O., Motrunich S., Cherniavska T., Papeikin O., Bodachivska L., Bardeau J.-F. Synthesis and evaluation of N,N-dibutylundecenamide as new eco-friendly plasticizer for polyvinyl chloride. Journal of Materials Science. 2022. V. 57. Р. 6102-6114. https://doi.org/10.1007/s10853-022-07006-0
28. Rogalsky S., Tarasyuk O., Dzhuzha O., Hodyna D., Cherniavska T., Hubina A., Filonenko M., Metelytsia L. Evaluation of N,N-dibutylolelamide as a bifunctional additive for poly(vinyl chloride). Colloid and Polymer Science. 2022. V. 300 (12). Р. 1-8. http://dx.doi.org/10.1007/s00396-022-05038-1
29. S.I. Vdovenko,I.I. Gerus,M. Pagacz-Kostrzewa,M. Wierzejewska. Influence of the features of the spatial and electronic structure of α-substituted β-ethoxyvinyl trifluoromethyl ketones and secondary amines on their reactivity. Journal of Molecular Structure. 2022. V. 1255. Р. 132417. https://doi.org/10.1016/j.molstruc.2022.132417

30. J. Liu, W. Lin,A.E. Sorochinsky,G. Butler,A. Landa, J. Han,V.A. Soloshonok. Successful trifluoromethoxy-containing pharmaceuticals and agrochemicals. Journal of Fluorine Chemistry. 2022. V. 257-258. Р. 109978. https://doi.org/10.1016/j.jfluchem.2022.109978

31. J. Han,J. Escorihuela,S. Fustero,A. Landa,V.A. Soloshonok, A. Sorochinsky. Asymmetric Michael addition in synthesis of β-substituted GABA derivatives. Molecules. 2022. V. 27. Р. 3797. https://doi.org/10.3390/molecules27123797
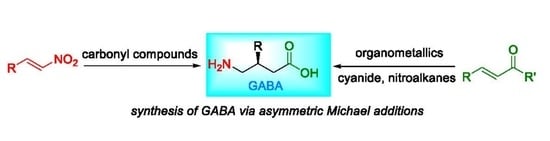
32. Q. Wang, J. Han, A. Sorochinsky, A. Landa, G. Butler, V. A. Soloshonok. The latest FDA-approved pharmaceuticals containing fragments of tailor-made amino acids and fluorine. Pharmaceuticals. 2022. V. 15. Р. 999. https://doi.org/10.3390/ph15080999
33. V.D. Romanenko. From elusive monomeric metaphosphates to oligomeric metaphosphate reagents:New avenue to halogen-free phosphorylation of biomolecules. Current Organic Chemistry. 2022. V. 26, Р. 432-437. https://doi.org/10.2174/1385272826666220330111824
34. Chernykh A.V.,Aloshyn D.,Kuchkovska Yu.O.,Daniliuc C.G.,Tolmachova N.A.,Kondratov I.S.,Zozulya S.,Grygorenko O.O., Haufe G. Impact of β-perfluoroalkyl substitution of proline on the proteolytic stability of its peptide derivatives Org. Biomol. Chem. 2022. V. 20. Р. 9337-9350. https://doi.org/10.1039/D2OB01430K
35. Logvinenko I.G.,Kondratov I.S.,Pridma S.O.,Tolmachova N.A.,Morev R.N.,Dolovanyuk V.G.,Boretsky A.L.,Stepaniuk R.O.,Trofymchuk S.A.,Mück-Lichtenfeld C., Daniliuc C.G.,Haufe G. Synthesis and physical chemical properties of CF3O-containg secondary amines – Perspective building blocks for drug discovery Journal of Fluorine Chemistry.2022. V. 257–258. Р. 109990. https://doi.org/10.1016/j.jfluchem.2022.109990

36. Homon A.A.,Shynder L.V.,Demchuk O.P.,Hryshchuk O.V.,Kondratov I.S.,Gerus I.I.,Grygorenko O.O. Synthesis of 1,3-bifunctional cyclobutane derivatives with α-CHF2/CF3 group – advanced building blocks for medicinal chemistry Journal of Fluorine Chemistry. 2022. V. 263. Р. 110041. https://doi.org/10.1016/j.jfluchem.2022.110041

37. Kondratov I.S.,Moroz Yu.S.,Irwin J.J.,Shoichet B.K. Drug building blocks and libraries at risk in Ukraine Science. 2022. V. 376(6596). Р. 929. https://doi.org/10.1126/science.abq7841
38. Kondratov I.S.,Moroz Yu.S.,Grygorenko O.O.,Tolmachev A.A. The Ukrainian Factor in Early-Stage Drug Discovery in the Context of Russian Invasion: The Case of Enamine Ltd ACS Med. Chem. Lett. 2022. V. 13. N 7. Р. 992-996. https://doi.org/10.1021/acsmedchemlett.2c00211

39. M. Aksylenko, E. Sheludko, N. Himach, V. Yevdokimenko. Polygalacturonates of biogenic metals – prospective components of new composite preparations for wheat. J. Annual Research & Review in Biology. 2022. V. 37 (12). P. 17-28. https://doi.org/10.9734/arrb/2022/v37i1294273
40. A.O. Kolodiazhna,O.I. Kolodiazhnyi Сatalytic asymmetric synthesis of C-chiral phosphonate Symmetry. 2022. V. 14. P. 1758-1825. https://doi.org/10.3390/sym14091758
41. D. Prysiazhnuk, A. Kolodiazhna, O. Kolodiazhnyi Сonvergent method for the determination of halo-2,3-dihydro-1H-inden-1-ol absolute configuration Arkivoc. 2022. V. IX. P. 23-32. https://doi.org/10.24820/ark.5550190.p011.835
42. N.A. Ogurtsov,A.V. Mamykin,O.L. Kukla,A.S. Pavluchenko,M.V. Borysenko,Yu.P. Piryatinski,J.-L. Wojkiewicz,A.A. Pud. The impact of interfacial interactions on structural, electronic and sensing properties of poly(3-methylthiophene) in the core-shell nanocomposites. Application to the CWA simulants detection. Macromolecular Materials and Engineering. 2022. V. 307(4). P. 2100762. http://dx.doi.org/10.1002/mame.202100762
43. T. Feng, Y. Yuan, X. Chen,S. Zhao, M. Cao, L. Feng,S. Shi, H. Wang,T. Liu, A. Pud,L. Han, R. Scaffaro, B. He, N. Wang. Ultrasensitive and highly specific detection of iodine ions using zirconium (IV)-enhanced oxidation Cell. Reports Physical Science. 2022. V. 3 (11). P. 101143 (10 pages). https://doi.org/10.1016/j.xcrp.2022.101143

44. Potikha L.M., Brovarets V.S., Zhirnov V.V. Anticancer evaluation of difunctional substituted 1,2-dihydrophthalazines Chem. Data Collect. 2022. V. 37. P. 100817. https://doi.org/10.1016/j.cdc.2021.100817

45. Demydchuk B.A., Mykhalchenko O.A., Rusanov E.B., Moskvina V.S., Brovarets V.S. Concise and regioselective synthesis of 5H-imidazo[1,2-e][1,3,5]triazepines. Arkivoc. 2022. V. ІІ. P. 204-214. http://dx.doi.org/10.24820/ark.5550190.p011.689
46. Nizhenkovska I.V., Matskevych K.V., Golovchenko O.I., Golovchenko O.V., Kustovska A.D., Van M. New prospective phosphodiesterase inhibitors: phosphorylated oxazole derivatives in treatment of hypertension. Adv. Pharm. Bull., 2022. https://doi.org/10.34172/apb.2023.044
47. Brusnakov M., Golovchenko O., Velihina Ye., Liavynets O., Zhirnov V., Brovarets V. Evaluation of anticancer activity of 1,3-oxazol-4-ylphosphonium salts in vitro Chem. Med. Chem. 2022. V. 17, № 20. P. e202200319. https://doi.org/10.1002/cmdc.202200319

48. Biletska I.M., Mrug G.P., Prostota Ya.O., Kondratyuk K.M., Bondarenko S.P., Frasinyuk M.S. Synthesis of 2-trifluoroacetonyl-3-alkyl/alkoxy-chromones and their reactions with 1,2-bidentate nucleophiles. Heterocycles. 2022. V. 104, N 7. P. 1229-1244. http://dx.doi.org/10.3987/COM-22-14659
49. Frasinyuk M., Chhabria D., Kartsev V., Dilip H., Sirakanyan S.N., Kirubakaran S., Petrou A., Geronikaki A., Spinelli D. Benzothiazole and chromone derivatives as potential ATR kinase inhibitors and anticancer agents. Molecules. 2022. V.27, N 14. P. 4637. https://doi.org/10.3390/molecules27144637
50. Waszkowska K., Krupka A., Smokal V., Kharchenko O., Migalska-Zalas A., Frasinyuk M., Wielgosz R., Andrushchak A., Sahraoui B. Correlation between nonlinear optical effects and structural features of aurone-based methacrylic polymeric thin films. Materials. 2022. V.15, N 17. P. 6076. https://doi.org/10.3390/ma15176076
51. Obernikhina N.V., Kachaeva M.V., Kachkovsky O.D., Brovarets V.S. In silico study of conjugated nitrogen heterocycles affinity in their biological complexes. Chem. Heterocycl. Comp. 2022. V. 58, N8/9. P. 412-420. https://doi.org/10.1007/s10593-022-03107-5

52. Kornii Yu., Shablykin O., Tarasiuk T., Stepaniuk O., Matvienko V., Aloshyn D., Zahorodniuk N., Sadkova I.V., Mykhailiuk P.K. Fluorinatedaliphatic diazirines: preparation, characterization, and model photolabeling studies. J. Org. Chem. 2022. https://doi.org/10.1021/acs.joc.2c02262

53. Glibov E.K., Gorbulenko N.V., Moskvina V.S., Suprun A.V., Shablykina O.V. Shokol T.V., Khilya V.P. Synthesis and recyclization of methylenbisflavonoids based on heterocyclic analogues of umbelliferon and formononetin Chem. Nat. Compd. 2022. V. 58, N 4. P. 617-622. https://doi.org/10.1007/s10600-022-03755-1
54. Konovalenko A.S., Shablykina O.V., Shablykin O.V., Moskvina V.S., Brovarets V.S. 1H-isochromene-1-ones and isoquinoline-1(2H)-ones with carbonyl group in position 3: Features of synthetic approaches and transformation. Arkivoc. 2022. V. VІІІ. P. 79-112. https://doi.org/10.24820/ark.5550190.p011.861
55. Potikha L.M., Brovarets V.S., Zhirnov V.V. Biological evaluation of 3-aminoisoquinolin-1(2H)-one derivatives as potential anticancer agents. French-Ukr. J. Chem. 2021. V.9, №2. P. 52-63. http://dx.doi.org/10.17721/fujcV9I2P52-63
56. A.V. Mamykin, O.L. Kukla, A.S. Pavluchenko, Z.I. Kazantseva, I.A. Koshets, A.A. Pud, N.A. Ogurtsov, Yu.V. Noskov, V.I. Kalchenko Electronic nose-type chemosensory systems for detection of gaseous poisonous substances. Semiconductor Physics, Quantum Electronics & Optoelectronics. 2022. V.25, No.4. Р. 429-440. https://doi.org/10.15407/spqeo25.04.429
57. V.D. Romanenko, J.-M. Sotiropoulos. Six-membered rings with two or more heteroatoms with at least one boron. Comprehensive Heterocyclic Chemistry IV, Elsevier. New York. 2022. Р.806-845. http://dx.doi.org/10.1016/B978-0-12-818655-8.00098-6
58. Grygorenko O.O., Hutskalova V., Moskvina V.S. Bicyclic 6-6 system with one bridgehead (ring junction) nitrogen atom: three extra heteroatoms (2:1).Chapter in book: “Comprehensive heterocyclic chemistry IV (Fourth edition)”, 2022. P. 216-278. https://doi.org/10.1016/b978-0-12-409547-2.14958-3.
1. Yakovlieva A., Boichenko S., Zubenko S., Konovalov S. Synthesis and physico-chemical properties of palm kernel oil bioadditives for alternative jet fuel. Systems and means of motor transport. Seleced problems. Seria: Transport. Monografia. Rzeszow (Poland). 2021. Р. 257-264.
2. I.G. Logvinenko, I.S. Kondratov, A.V. Dobrydnev, A.V. Kozytskiy, O.O. Grygorenko. Synthesis and reactions of ω-CF3O-substituted aliphatic sulfonyl chlorides. Journal of Fluorine Chemistry. 2021. V. 246. Р. 109799. https://doi.org/10.1016/j.jfluchem.2021.109799

3. J. Han, N. Lyutenko, A. Sorochinsky, A. Okawara, H. Konno, S. White, V. Soloshonok. Tailor-Made Amino Acids in Pharmaceutical Industry: Synthetic Approaches to aza-tryptophane derivatives. Chem. Eur. J. 2021. https://doi.org/10.1002/chem.202102485

4. E.N. Shaitanova, O.A. Balabon, A.N. Rybakova, T.S. Khlebnicova, F.А. Lakhvich, I.I. Gerus. Synthesis of functionalized fluoroalkyl pyrimidines and pyrazoles from fluoroalkyl enones. Journal of Fluorine Chemistry. 2021. V. 252. Р. 109905 https://doi.org/10.1016/j.jfluchem.2021.109905

5. A.A. Homon, O.V. Hryshchuk, O.V. Mykhailenko, B.V. Vashchenko, K.P. Melnykov, O.M. Michurin, C.G. Daniliuc, I.I. Gerus, V.O. Kovtunenko, I.S. Kondratov, O.O. Grygorenko. 4-(Di‐/Trifluoromethyl)‐2‐heterabicyclo[2.1. 1]- hexanes: advanced fluorinated phenyl isosteres and proline analogues. European Journal of Organic Chemistry. 2021 https://doi.org/10.1002/ejoc.202100414

6. S. Trofymchuk, M. Bugera, A. Klipkov, V. Ahunovych, B. Razhyk, S. Semenov, A. Boretskyi, K. Tarasenko, P. Mykhailiuk. Scalable Approach to Fluorinated Heterocycles with Sulfur Tetrafluoride (SF4). J. Org. Chem. 2021. V. 86, № 17. Р. 12181-12198 https://doi.org/10.1021/acs.joc.1c01518

7. V.D. Romanenko. New trends in development of P-C bond forming reactions. Current Organic Chemistry. 2021. V.25, № 17. Р. 1937-1976. https://doi.org/10.2174/1385272825666210610153954
8. Noskov Yu., Ogurtsov N., Bliznyuk V., Lvov Yu., Myronyuk I., Pud A. Synthesis and properties of coreshell halloysite–polyaniline nanocomposites. Applied Nanoscience. 2021. V. 12, Р. 1285–1294 https://doi.org/10.1007/s13204-021-01812-9
9. O.I. Kolodiazhnyi. Phosphorus Compounds of Natural Origin: Prebiotic, Stereochemistry, Application. Symmetry. 2021. V. 13(5)., № 889. Р. 1-52. https://doi.org/10.3390/sym13050889
10. D.V. Prysiazhnuk, E.B. Rusanov, O.I. Kolodiazhnyi. The absolute configuration of 2-bromo-2,3-dihydro-1H-inden-1-ols. Synthetic communications. 2021. V. 51, № 19. Р. 3023–3031. https://doi.org/10.1080/00397911.2021.1960378

11. O.O. Kolodiazhna, D.V. Prysiazhnuk, A.O. Kolodiazhna, O.I. Kolodiazhnyi. Synthesis of optically active vicinal fluorocyclopentanols and fluorocyclopentanamines by enzymatic deracemization. Arkivoc 2022, p. iii. Р. 14-26 https://doi.org/10.24820/ark.5550190.p011.634
12. O. Kolodiazhnyi, A. Kolodiazhna, E. Grishkun, D. Prysiazhnuk, O. Kolodiazhna, S. Sheiko. Achievements in developments of stereochemistry. Phosphorus, sulfur, and silicon and the related elements. 2021. Р. 474-479. https://doi.org/10.1080/10426507.2021.2011874

13. A. Kolodiazhna, D. Prysiazhnuk, O. Kolodiazhnyi. Asymmetric Electrophilic Reactions in Phosphorus Chemistry.Phosphorus, sulfur, and silicon and the related elements. 2021. V. 196. Р. 505-507. https://doi.org/10.1080/10426507.2021.1989687

14. A. Kolodiazhna, E. Grishkun, O. Kolodiazhnyi. Synthesis of chiral phosphonobenzaldehydes and phosphonotyrosine. Phosphorus, sulfur, and silicon and the related elements 2021, 196. Р. 502-504. https://doi.org/10.1080/10426507.2021.1989686

15. Metelytsia L., Hodyna D., Dobrodub I., Semenyuta I., Zavhorodnii M., Blagodatny V., Kovalishyn V., Brazhko O. Design of (quinolin-4-ylthio)carboxylic acids as new Escherichia coli DNA gyrase B inhibitors: machine learning studies, molecular docking, synthesis and biological testing. Comput. Biol. Chem. 2020. V. 24, № 85. Р. 107224 https://doi.org/10.1016/j.compbiolchem.2020.107224

16. Metelytsia L.O., Trush M.M., Kovalishyn V.V., Hodyna D.M., Kachaeva M.V., Brovaret V.S., Pilyo S.G., Sukhoveev V.V., Tsyhankov S.A., Blagodatnyi V.M., Semenyuta I.V. 1,3-Oxazole derivatives of cytisine as potential inhibitors of glutathione reductase of Candida spp.: QSAR modeling, docking analysis and experimental study of new anti-Candida agents. Comput. Biol. Chem. 2021. V. 90. Р. 107407. https://doi.org/10.1016/j.compbiolchem.2020.107407
17. Hodyna D., Kovalishyn V., Semenyuta I., Blagodatny V., Rogalsky S., Metelytsia L. In silico and in vitro Studies of Imidazolium Ionic Liquids as Effective Antibacterial Agents against Multidrug Resistant Escherichia coli Strains.. Current Bioactive Comp. 2021. V. 17, № 2. P. 130–144. https://doi.org/10.2174/1573407216999200422115655

18. Semenyuta I., Trush M., Kovalishyn V., Rogalsky S., Hodyna D., Karpov P., Xia Z., Tetko I., Metelytsia L. Structure-Activity Relationship Modeling and Experimental Validation of the Imidazolium and Pyridinium Based Ionic Liquids as Potential Antibacterials of MDR Acinetobacter Baumannii and Staphylococcus Aureus. Int. J. Mol. Sci. 2021. V.22. Р. 563-576. https://doi.org/10.3390/ijms22020563
19. Tsygankova V.A.Characterisation of Endo-Polygalacturonases activities of Rice (Oryza sativa) Fungal Pathogens in Nigeria, West Africa.Chapter in book: Grain and Seed Proteins Functionality (Jimenez-Lopez, J.C. Ed). Chapter 10. 2021. Intechopen Limited, London, United Kingdom. DOI: 10.5772/intechopen.94763.
20. T.Tkachenko, Ye.Sheludko, V.Yevdokymenko, D.Kamenskyh, N. Khimach, V. Povazhny, M. Filonenko, M. Aksylenko, V. Kashkovsky. Physico-chemical properties of flax microcrystalline cellulose. Applied Nanoscience (2021). Р.1007–1020. https://doi.org/10.1007/s13204-021-01819-2
21. O. Pertko, Yu.Voloshyna, A. Kontsevoi, V.Trachevskyi. Ethylbenzene formation and its conversion towards coke in the side-chain methylation of toluene on a basic X zeolite. Journal of Porous Materials. 2021. V.28. P. 1713–1723. https://doi.org/10.1007/s10934-021-01119-8.
22. L.K. Patrylak, O.P. Pertko, V.A. Povazhnyi, A.V. Yakovenko, S.V. Konovalov. Evaluation of nickel-containing zeolites in the catalytic transformation of glucose in an aqueous medium. Applied Nanoscience. 2021. Р. 869–882. https://doi.org/10.1007/s13204-021-01771-1
23. L.K. Patrylak, O.P. Pertko, A.V. Yakovenko, Yu.G. Voloshyna, V.A. Povazhnyi, M.M. Kurmach. Isomerization of linear hexane over acid-modified nanosized nickel-containing natural Ukrainian zeolites. Applied Nanoscience. 2021. Р. 411–425. https://doi.org/10.1007/s13204-021-01682-1
24. Kalishyn Ye., Bychko I., Kameneva T., Skoblik O., Polunkin Ye., Strizhak P. Inhibition effect of the α-FeOOH nanoparticles in the benzyl alcohol oxidation.Journal of Cluster Science. 2021. Р. 1337–1343. https://doi.org/10.1007/s10876-021-02056-x
25. Shatursky O.Ya., Manoilov K.Yu., Gorbatiuk O.B., Usenko M.O., Zhukova D.A., Vovk A.I., Kobzar O.L., Trikash I.O., Borisova T.A., Kolibo D.V., Komisarenko S.V. The geometry of diphtheria toxoid CRM197 channel determined with thiazolium salts and nonelectrolytes. Biophys. J. 2021. V. 120, I. 12. P. 2577-2591. https://doi.org/10.1016/j.bpj.2021.04.028
26. Kobzar О. L., Shulha Y. V., Buldenko V. M., Mrug G. P., Kolotylo M. V., Stanko O. V., Onysko P.P., Vovk А. I. Alkyl and aryl α-ketophosphonate derivatives as photoactive compounds targeting glutathione-S-transferases. Phosphorus, Sulfur, and Silicon and the Related Elements. 2021. V. 196 (7). Р. 672-678. https://doi.org/10.1080/10426507.2021.1901703

27. Rogalsky S., Bardeau J.-Fr., Lyoshina L., Bulko O., Tarasyuk O., Dzhuzha O., Cherniavska T., Kremenitsky V., Kobrina L., Riabov S. New promising antimicrobial material based on thermoplastic polyurethane modified with polymeric biocide polyhexamethylene guanidine hydrochloride. Materials Chemistry and Physics. 2021. V.267. Р. 124682. https://doi.org/10.1016/j.matchemphys.2021.124682
28. Orlovska I., Podolich O., Kukharenko O., Zaets I., Reva O., Khirunenko L., Zmejkoski D., Rogalsky S., Barh D., Tiwari S., Kumavath R., Góes-Neto A., Azevedo V., Brenig B., Ghosh P., de Vera J.-P., Kozyrovska N. Bacterial Cellulose Retains Robustness but Its Synthesis Declines After Exposure to a Mars-Like Environment Simulated Outside the International Space Station. Astrobiology. 2021. V. 21 (7). P. 706-717. http://dx.doi.org/10.1089/ast.2020.2332
29. Rogalsky S., Bardeau J.-Fr., Makhno S., Tarasyuk O., Babkina N., Cherniavska T., Filonenko M., Fatyeyeva K. New polymer-electrolyte membrane for medium-temperature fuel cell applications based on cross-linked polyimide Matrimid and hydrophobic protic ionic liquid. Materials Today Chemistry. 2021. V. 20. P. 100453. https://doi.org/10.1016/j.mtchem.2021.100453
30. L. Muzychka, A. Voronkina, V. Kovalchuk, O. Smolii, M. Wysokowski, I. Petrenko, D. Youssef, I. Ehrlich, H. Ehrlich. Marine biomimetics: bromotyrosines loaded chitinous skeleton as source of antibacterial agents. Appl. Phys. A Mater. Sci Process. 2021. V. 127, № 1. P. 15. https://doi.org/10.1007/s00339-020-04167-0
31. L.V. Muzychka, E.V. Verves, I.O. Yaremchuk, A.M. Zinchenko, S.V. Shishkina, I.V. Semenyuta, D.M. Hodyna, L.O. Metelytsia, V.Kovalishyn, O.B. Smoliі. Synthesis, QSAR modeling, and molecular docking of novel fused 7-deazaxanthine derivatives as adenosine A2A receptor antagonists. Chem. Biol. Drug Des. 2021. V. 98. P. 1-8. https://doi.org/10.1111/cbdd.13975
32. Vydzhak R.N., Panchishin S.Ya., Kachaeva M.V., Pilyo S.G., Moskvina V.S., Shablykina O.V., Kozytskiy A.V., Brovarets V.S. A rapid synthetic approaches to the libraries of diversified 1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones and 3-(2-hydroxyphenyl)-4,5-dihydropyrrolo[3,4-c]pyrazole-6(1H)-ones. Molecular Diversity. 2021. Р. 1115–1128. https://doi.org/10.1007/s11030-021-10234-2

33. Shcherbakova V., Dibchak D., Snisarenko M., Skalenko Ye., Denisenko A.V., Kuznetsova A.S., Mykhailiuk P.K. Bicyclic piperidines via [2+2] photocycloaddition. J. Org. Chem. 2021. V.86, №3. P. 2200-2209. https://doi.org/10.1021/acs.joc.0c02355

34. Slyvchuk S., Pilyo S., Brovarets V., Kartsev V., Angeli A., Pinteala M., Petrou A., Geronikaki A., Supuran C. Chromene-containing aromatic sulfonamides with carbonic anhydrase inhibitory properties.Int. J. Mol. Sci. 2021. V.22, №10. P. 5082 https://doi.org/10.3390/ijms22105082
35. Zhirnov V.V., Velihina Ye.S., Mitiukhin O.P., Brovarets V.S. Intrinsic drug potential of oxazolo[5,4-d]pyrimidines and oxazolo[4,5-d]pyrimidines.Chem. Biol. Drug Des. 2021. V.98, № 4. P. 561-581. https://doi.org/10.1111/cbdd.13911
36. Merzhyievskyi D.O., Shablykin O.V., Shablykin O.V, Kozytskiy A.V., Rusanov E.B., Moskvina V.S., Brovarets V.S. Functionalized 5-amino-4-cyanooxazoles, their hetero and macrocyclic derivatives: preparation and synthetic applications. Eur. J. Org. Chem. 2021. https://doi.org/10.1002/ejoc.202100412

37. Angeli A., Kartsev V., Petrou A., Pinteala M., Vydzhak R., Panchishin S., Brovarets V., de Luca V., Capasso C., Geronikaki A., Supuran C. New sulfanilamide derivatives in corporating heterocyclic carboxamide moieties as carbonic anhydrase inhibitors. Pharmaceuticals. 2021. V. 14, № 8. P. 828. https://doi.org/10.3390/ph14080828
38. Kovalishyn V., Zyabrev V., Kachaeva M., Ziabrev K., Keith K., Harden E., Hartline C., James S., Brovarets V. Design of new imidazole derivatives with anti-HCMV activity: QSAR modeling, synthesis and biological testing.J. Comp.-Aided Mol. Des. 2021. V.35. P.1177-1187. doi.org/10.1007/s10822-021-00428-z
39. Velihina E.S., Obernikhina N.V., Pilyo S.G., Kachkovsky O.D., Brovarets V.S. Synhesis, electronic structure and anti-cancer activity of the phenyl substituted pyrazolo[1,5-a][1,3,5]triazines. Current Org. Chem. 2021. V.25, №12. P. 1441-1454. http://dx.doi.org/10.2174/1385272825666210607004536

40. Maiko K., Merzhyievskyi D., Piryatinski Yu., Obernikhina N., Prostota Ya., Dmitruk I., Kachkovsky O., Brovarets V. Study of excited state relaxation by time-resolved spectroscopy in conjuigated substituted polyene bis-oxazoles. Structural Chem. 2021. V.32, №3. P. 977-987. https://doi.org/10.1007/s11224-021-01752-8
41. Angeli A., Kartsev V., Petrov A., Pinteala M., Brovarets V., Panchishin S., Vydzhak R., Geronikaki A., Supuran C.T. Carbonic anhydrase inhibition with sulfonamides incorporatiny pyrazole- and pyridazinecarboxamide moieties provides examples of isoform-selective inhibitors. Molecules. 2021. V. 26, № 22. P. 7023-7043. https://doi.org/10.3390/molecules26227023
42. Ostapiuk Yu.V., Shehedyn M., Barabash O.V., Demydchuk B.A., Batsyts S., Herzberer C., Schmidt A. Bromoarylation of methyl 2-chloroacrylate under Meerwein conditions for the synthesis of substituted 3-hydroxyhiophenes. Synthesis. 2021. Р. 732-740. https://doi.org/10.1055/s-0040-1719849

43. Shablykina O.V., Shylin S.V., Moskvina V.S., Ischenko V.V., Khilya V.P. Progress in the chemistry of aminoacid derivatives of isocoumarines and 3,4-dihydroisocoumarines.Chem. Nat. Compd. 2021. V. 57, № 2. P. 209-229. https://doi.org/10.1007/s10600-021-03323-z
44. Biletska I.M., Mrug G.P., Bondarenko S.P., Kondratyuk K.M., Prostota Y.O., Sviripa V.M., Frasinyuk M.S. Chemoselective synthesis of 3-trifluoromethylpyrazole-deoxybenzoin hybrids. J. Fluorine Chem. 2021. V.242. P. 109698. https://doi.org/10.1016/j.jfluchem.2020.109698

45. Bondarenko S.P., Mrug G.P., Vinogradova V.I., Frasinyuk M.S. Synthesis of new conjugates of coumarins with anabasine and cytisine. Chem. Nat. Compd. 2021. V. 57, № 1. P. 9-13. https://doi.org/10.1007/s10600-021-03268-3
46. Shokol T.V., Moskvina V.S., Hlibov Y. K., Frasinyuk M.S., Khilya V.P. Synthesis of furoneoflavones modified by coumarin and (het)aroyl substituents. Chem. Nat. Compd. 2021. V. 57, № 1. P. 33-37. https://doi.org/10.1007/s10600-021-03275-4
47. Piryatinski Yu.P., Verbitsky A.B., Dmytruk A., Malynovskyi M.B., Lutsyk P.M., Rozhin A.G., Kachkovsky O.D., Prostota Ya.O., Kurdyukov V.V. Excited state relaxation in cationic pentamethine cyanines studied by time-resolved spectroscopy.Dyes Pigm. 2021. V. 193. P. 109539. https://doi.org/10.1016/j.dyepig.2021.109539.

48. Navozenko O., Yashchuk V., Kachkovsky O., Gudeika D., Butkute R., Slominskii Yu., Azovskyi V. Aggregate formation of boron-containing molecules in thermal vacuum deposited films. Materials. 2021. V. 14, №19. P. 5615. https://doi.org/10.3390/ma14195615.
49. Bashmakova N.V., Shaydyuk Ye.O., Dmytruk A.M., Swiergosz T., Kachkovsky O.D., Belfield K.D., Bondar M.V., Kasprzyk W. Nature of linear spectral properties and fast electronic relaxations in green fluorescent pyrrolo[3,4-c]pyridine derivative.Int. J. Mol. Sci. 2021. Vol. 22, №11. P. 5592. https://doi.org/10.3390/ijms22115592.
50. Shaydyuk Ye.O., Bashmakova N.V., Dmytruk A.M., Kachkovsky O.D., Koniev S., Strizhak A.V., Komarov I.V., Belfield K.D., Bondar M.V., Babii O. Nature of fast relaxation processes and spectroscopy of a membrane-active peptide modified with fluorescent amino acid exhibiting excited state intramolecular proton transfer and efficient stimulated emission.ACS Omega. 2021. V. 6, № 15. P. 10119-10128. https://doi.org/10.1021/acsomega.1c00193.

51. Vasylyuk S.V., Suprun A.D., Shmeleva L.V., Kachkovsky O.D. Configuration of charge waves in polymethine linear dye systems. Springer proceedings in physics. 2021. V. 246. P. 189-201. https://doi.org/10.1007/978-3-030-51905-6_15
52. M.D. Aksylenko, V.A. Yevdokymenko, T.V. Tkachenko, D.S. Kamenskyh, V.I. Kashkovsky. Effective organo-mineral fertilizers of prolonged action from the processed organo-containing wastes of various origin. Proceeding Book of III Balkan agricultural congress. August 30-September 1, 2021. Edirne, Turkey. P. 554-564. https://www.cabidigitallibrary.org/doi/pdf/10.5555/20220174092
53. V. Bratishko, T. Tkachenko, S. Shulha, O. Tigunova. Results of composition analysis of non-grain part of major field crops in Ukraine. Proceeding Book of 20th International Scientific Conference Еngineering for rural development. V. 20. May 26-28, 2021. Jelgava, Latvia. Р. 584-588. https://doi.org/10.22616/ERDev.2021.20.TF125.
54. Starodubtseva A., Kalachova T., Iakovenko O., Stoudková V., Zhabinskii V., Khripach V., Ruelland E., Martinec J., Burketová L., Kravets V. BODIPY Conjugate of Epibrassinolide as a Novel Biologically Active Probe for іn vivo Imaging. International Journal of Molecular Sciences. 2021. V. 22 (3599). Р. 1-12. https://doi.org/10.3390/ijms22073599
55. Pokotylo I., Hodges M., Kravets V., Ruelland E. A ménage à trois: Salicylic acid, growth inhibition and immunity. Trends in plant Sciences. 2021. TRPLSC2223 https://doi.org/10.1016/j.tplants.2021.11.008.
56. O.O. Grygorenko, V.S. Moskvina, I. Kleban, O.V. Hryshchyk. Synthesis of saturated and partially saturated heterocyclic boronic derivatives. Tetrahedron. 2021. https://doi.org/10.1016/j.tet.2021.132605.

1. Romanenko V.D. Rings containing group 15 elements. Ref. Mod. Chem. Molecular Sciences. Elsevier. 2020. Р.1-36.
2. Parkhomenko Y., Vovk A., Protasova Z. Vitamin B1 and the pyruvate dehydrogenase complex. In Molecular Nutrition. Vitamins. Academic Press. 2020. P. 185-206. https://doi.org/10.1016/B978-0-12-811907-5.00012-9

3. Poda G., Tanchuk V. Computational Methods for the Discovery of Chemical Probes.The Discovery and Utility of Chemical Probes in Target Discovery. RSC Publishing. 2020. P. 39-68. http://dx.doi.org/10.1039/9781839160745-00039

4. V.A. Tsygankova, K.B. Blyuss, E.N. Shysha, L.A. Biliavska, G.A. Iutynska, Y.V. Andrusevich, S.P. Ponomarenko, A.I. Yemets, Y.B. Blume. Using Microbial Biostimulants to Deliver RNA Interference in Plants as an Effective Tool for Biocontrol of Pathogenic Fungi, Parasitic Nematodes and Insects. In Monograph «Research Advances in Plant Biotechnology». Series: Plant Science Research and Practices. Chapter 6. Nova Science Publishers, Inc. USA. 2020. 375 p. P. 205-319.
5. A.O. Adejuwon, V.A. Tsygankova. α-Amylase Production by Toxigenic Strains of Aspergillus and Penicillium.In Monograph “Aflatoxin B1 Occurrence, Detection and Toxicological Effects” Ed. by Xi-Dai Long. IntechOpen, 2020. P. 1-22. DOI: 10.5772/intechopen.86637
6. A.О. Kolodiazhna, O.I. Kolodiazhnyi. Asymmetric Electrophilic Reactions in Phosphorus Chemistry. Symmetry. 2020. V.12, № 1. Р. 108-159. https://doi.org/10.3390/sym12010108
7. A.О. Kolodiazhna, А.I Skliarov, A.A. Slastennikova, O.I Kolodiazhnyi. Asymmetric Synthesis of (S,R)- and (R,R)-Methiin Stereoisomers. Phosph., Sulf., Silicon and Relat. Elem. 2020. V.195. P. 713-717. http://dx.doi.org/10.1080/10426507.2020.1755972

8. T. Tkachenko, V. Yevdokymenko, D. Kamenskyh, Y. Sheludko, V. Povazhny, V. Kashkovsky. Physico-chemical properties of biogenic SiO2 nanoparticles obtained from agriculture residue. Applied Nanoscience. 2020. V. 10, № 12. P. 4617 – 4623. https://doi.org/10.1007/s13204-020-01383-1
9. Tigunova O.O., Kamenskyh D.S., Tkachenko T.V., Yevdokymenko V.A., Kashkovskiy V.I., Rakhmetov D.B., Blume Ya.B., Shulga S.M.. Biobutanol Production from Plant Biomass. The Open Agriculture Journal. 2020. V. 14. P. 187-197. https://doi.org/10.2174/1874331502014010187
10. V. Kovalishyn, D. Hodyna, V.O. Sinenko, V. Blagodatny, I. Semenyuta, S. R. Slivchuk, V. Brovarets, G. Poda, L. Metelytsia. Hybrid Design of Isonicotinic Acid Hydrazide Derivatives: Machine Learning Studies, Synthesis and Biological Evaluation of their Antituberculosis Activity. Current Drug Discovery Technologies. 2020. V. 17. Р. 365-375. https://doi.org/10.2174/1570163816666190411110331
11. M. Trush, V. Kovalishyn, D. Hodyna O. Golovchenko S. Chumachenko I. Tetko V. Brovarets L. Metelytsia. In silico and in vitro studies of a number PILs as new antibacterials against MDR clinical isolate Acinetobacter baumannii. Chem Biol Drug Des. 2020. V. 95. Р. 624-630. https://doi.org/10.1111/cbdd.13678
12. N. Abramenko, L. Kustova, L. Metelytsia, V. Kovalishyn, I. Tetko, W. Peijnenburg. A review of recent advances towards the development of QSAR models for toxicity assessment of ionic liquids. Journal of Hazardous Materials. 2020. V. 384. 121489. Р. 1-14. https://doi.org/10.1016/j.jhazmat.2019.121429
13. M. M.Trush, I. Semenyuta, D. Hodyna, A. Ocheretniuk, S. Vdovenko, S. Rogalsky, L. Kalashnikova, V. Blagodatnyi, O. Kobzar, L. Metelytsia. Functionalized imidazolium-based ionic liquids: biological activity evaluation,toxicity screening, spectroscopic, and molecular docking studies. Medicinal Chemistry Research. 2020. V. 29. Р. 2181-2191. https://doi.org/10.1007/s00044-020-02631-3
14. L. Metelytsia, M. Trush, I. Semenyuta, S. Rogalsky, O. Kobzar, L. Kalashnikova, V. Blagodatny, D. Hodyna. Ionic Liquids with Anti-Candida and Anticancer Dual Activity as Potential N-Myristoyltransferase Inhibitors. Current Bioactive Compounds. 2020. V.16, №7. Р. 1036-1041. https://doi.org/10.2174/1573407215666191007120402
15. Ghamrawi S., Bouchara J.-Ph., Corbin A., Rogalsky S., Tarasyuk O., Bardeau J.-Fr. Inhibition of fungal growth by silicones modified with cationic biocides. Materials Today Communications. 2020. V. 22. Р. 100716. https://doi.org/10.1016/j.mtcomm.2019.100716

16. Fatyeyeva, K., Rogalsky, S., Makhno, S., Tarasyuk, O., Soto Puente, J.A. Marais, S. Polyimide/Ionic Liquid Composite Membranes for Middle and High Temperature Fuel Cell Application: Water Sorption Behavior and Proton Conductivity. Membranes.2020. V.10. Р.82. https://doi.org/10.3390/membranes10050082
17. Pokotylo I., Hellal D., Bouceba T., Hernandez-Martinez M., Kravets V., Leitao L., Espinasse C., Kleiner I., Ruelland E.. Deciphering the Binding of Salicylic Acid to Arabidopsis thaliana Chloroplastic GAPDH-A1. International Journal of Molecular Sciences. 2020. V. 21, №13. Р. 4678. https://doi.org/10.3390/ijms21134678
18. Logvinenko I.G., Markushyna Y., Kondratov I.S., Vashchenko B.V., Kliachyna M., Tokaryeva Yu., Pivnytska V., Grygorenko O. O., Haufe G.. Synthesis, physico-chemical properties and microsomal stability of compounds bearing aliphatic trifluoromethoxy group. Journal of Fluorine Chemistry. 2020. V. 231. Р. 109461. https://doi.org/10.1016/j.jfluchem.2020.109461
19. Bugera M.Ya, Tarasenko K.V., Kondratov I.S., Gerus I. I, Vashchenko B.V., Ivasyshyn V.E. Grygorenko O.O.. (Het)aryl difluoromethyl-substituted β-alkoxyenones: synthesis and heterocyclizations. European Journal of Organic Chemistry. 2020. V. 9. Р. 1069-1077. https://doi.org/10.1002/ejoc.201901833

20. Malashchuk A., Chernykh, A.V., Hurmach V.V., Platonov M.O., Onopchenko O., Zozulya S., Daniliuc C.G., Dobrydnev A.V., Kondratov I.S., Moroz Yu.S. Grygorenko O.O. Synthesis, biological evaluation, and modeling studies of 1,3- disubstituted cyclobutane-containing analogs of combretastatin A4. Journal of Molecular Structure. 2020. V. 1210. Р. 128025-128033. https://doi.org/10.1016/j.molstruc.2020.128025

21. Stepaniuk O.O., Vashchenko B.V., Matvienko V.O., Kondratov I.S., Tolmachev A.A., Grygorenko O.O. Reactions of cyclic β-alkoxyvinyl α-keto esters with heteroaromatic NCC-binucleophiles. Chemistry of Heterocyclic Compounds. 2020. V. 56, № 3. Р. 377-385. https://doi.org/10.1007/s10593-020-02670-z
22. Stepaniuk O.O, Rudenko T.V., Vashchenko B.V., Matvienko V.O., Kondratov I.S., Tolmachev A.A., Grygorenko O.O. Synthesis of Fused Pyridine Carboxylates by Reaction of β-Alkoxyvinyl Glyoxylates with Amino Heterocycles. Synthesis. 2020. V. 52, № 13. Р. 1915-1926. DOI: 10.1055/s-0039-1707987

23. Feskov I.O., Golub B.O., Vashchenko B.V, Levterov V. V., Kondratov I.S., Grygorenko O.O., Haufe G.. GABA Analogues and Related Mono‐/Bifunctional Building Blocks Derived from the Fluorocyclobutane Scaffold. European Journal of Organic Chemistry. 2020. V. 30. Р. 4755-4767. https://doi.org/10.1002/ejoc.202000733

24. Klipkov A.A., Sorochinsky A.E., Tarasenko K.V., Rusanova J.A., Gerus I.I. Synthesis of trifluoromethyl and trifluoroacetyl substituted dihydropyrrolizines and tetrahydroindolizines. Tetrahedron Lett. 2020. V. 61. P. 151633. https://doi.org/10.1016/j.tetlet.2020.151633

25. Gerus I.I, Zhuk Y.I, Kacharova L.M, Röschenthaler G., Shaitanova E.N., Sorochinskii A.E., Vdovenko S. I., Wojcik Y.. Uncommon fluorination of enones with xenon difluoride. Journal of Fluorine Chemistry. 2020. V. 229. Р. 109413. https://doi.org/10.1016/j.jfluchem.2019.109413

26. Trofymchuk S., Bugera M., Klipkov A., Razhyk B., Semenov S., Tarasenko K., Starova V., Zaporozhets O., Tananaiko O., Alekseenko A., Pustovit Y., Kiriakov O., Gerus I., Tolmachev A., Mykhailiuk P.. Deoxofluorination of (Hetero) aromatic Acids. The Journal of Organic Chemistry. 2020. V. 85, № 5. Р. 3110-3124. https://doi.org/10.1021/acs.joc.9b03011

27. Trofymchuk S.A, Kliukovskyi D.V., Semenov S.V., Khairulin A.R., Shevchenko V.O., Bugera M.Y., Tarasenko K.V., Volochnyuk D.M., Ryabukhin S.V. Semi-Industrial Fluorination of β-Keto Esters with SF4: Safety vs Efficacy. Synlett. 2020. V. 31, № 06. Р. 565-574. https://doi.org/10.1055/S-0037-1610744
28. Vretik L.O., Noskov Y.V., Ogurtsov N.A., Nikolaeva O.A., Shevchenko A.V., Marynin A.I., Kharchuk M.S., Chepurna O.M., Ohulchanskyy T.Y., Pud A.A. Thermosensitive ternary core–shell nanocomposites of polystyrene, poly(N-isopropylacrylamide) and polyaniline. Applied Nanoscience. 2020. V. 10, № 12. P. 4951-4964. http://dx.doi.org/10.1007/s13204-020-01424-9
29. Rudenko R.M., Voitsihovska O.O., Poroshin V.M., Petrychuk M.V., Pavlyuk S.P., Nikolenko A.S., Ogurtsov N.A., Noskov Y., Sydorov D.O., Pud A.A.. Specific interactions and charge transport in ternary PVDF/polyaniline/MWCNT nanocomposite films(Article). Composites Science and Technology. 2020. V. 198. Р. 108284. https://doi.org/10.1016/j.compscitech.2020.108284

30. Kislyuk V., Kotrechko S., Trachevskij V., Melnyk A., Pud A., Ogurtsov N., Noskov Y., Osiponok M., Lytvyn P., Dzyazko Y., Akhmadaliev S., Kentsch U., Krause M., Facsko S. Impact of low energy ion beams on the properties of rr-P3HT films(Article). Applied Surface Science. 1 January 2021. V. 535. Р. 147619. http://dx.doi.org/10.1016/j.apsusc.2020.147619

31. Hamouda Z., Wojkiewicz J.L., Pud A.A., Bergheul S., Lasri T.. Broadband dielectric characterization of flexible substrates using organic conductive polymer microstrip lines. Microwave and Optical Technology Letters. 2020. V. 62, № 2. P. 688-695. https://doi.org/10.1002/mop.32110
32. Binnewerg B., Schubert M., Voronkina A., Muzychka L., Wysokowski M., Petrenko Ia., Djurović M., Kovalchuk V., Tsurkan M., Smolii O.B., Ehrlich H.. Marine biomaterials: Biomimetic and pharmacological potential of cultivated Aplysina aerophoba marine demosponge. Materials Science and Engineering C. 2020. V. 109. Р. 110566. https://doi.org/10.1016/j.msec.2019.110566
33. Mezhenska O., Rebriev A., Kobzar O., Zlatoust N., Vovk A., Parkhomenko Yu. Non-coenzyme properties of thiamine: evaluation of binding affinity to malate dehydrogenase isoforms. Biotechnologia Acta. 2020. V. 13, № 4. P. 26-38. https://doi.org/10.15407/biotech13.04.026
34. Shokol T.V., Gorbulenko N.V., Frasinyuk M.S., Khilya V.P.. Synthesis of 7-Hydroxy-8-Methyl-4′-Methoxy-6-Formylisoflavone and Linear Hetarenochromones Based on It. Chem. Nat. Compd. 2020. V.56, № 3. P. 420-422. https://doi.org/10.1007/s10600-020-03052-9
35. Tang B., Frasinyuk M.S., Chikwana V.M., Mahalingan K.K., Morgan C.A., Segvich D.M., Bondarenko S.P., Mrug G.P., Wyrebek P., Watt D.S., DePaoli-Roach A.A., Roach P.J., Hurley T.D. Discovery and Development of Small-Molecule Inhibitors of Glycogen Synthase. J. Med. Chem. 2020. V.63, № 7. P. 3538-3551. https://doi.org/10.1021/acs.jmedchem.9b01851

36. Shokol T.V., Gorbulenko N.V., Frasinyuk M.S., Khilya V.P. Synthesis of Benzofurans Modified by Coumarin and Pyrazole Heterocycles. Chem. Nat. Compd., 2020. V.56. P. 1060-1063. https://doi.org/10.1007/s10600-020-03226-5
37. Bondarenko S.P., Makarenko O.G., Vinogradova V.I., Frasinyuk M.S. Synthesis of 7-(N-12-Cytisinylpropoxy)Isoflavones. Chem. Nat. Compd. 2020. V.56 P. 1040-1043. https://doi.org/10.1007/s10600-020-03222-9
38. Golovchenko O.V., Abdurakhmanova E.R., Vladimirov S.O., Brusnakov M.Y., Krupoder T.O., Sukhoveev V.V., Rusanov E.B., Vydzhak R.N., Brovarets V.S. Interaction of 1-acylamino-2,2-dichloroethenyl(triphenyl)phosphonium chlorides with alkanolamines. Phosph. Sulph. Silicon and Relat. Elem. 2020. V.195, №10. P. 848-857. https://doi.org/10.1080/10426507.2020.1759062

39. Konovalenko A.S., Shablykin O.V., Brovarets V.S., Shablykina O.V., Moskvina V.S., Kozytskiy A.V.. 3-Hetarylisocoumarins in the synthesis of 1‑functionalized 3-hetarylisoquinolines. Chem. Het. Compd. 2020. V.56, № 8. P. 1021-1029. https://doi.org/10.1007/s10593-020-02769-3
40. Omelian T.V., Ostapchuk E.N., Dobrydnev A.V., Malets Y.S., Brovarets V.S., Grygorenko O.O. Strategy for the synthesis of 2,2-disubstituted 8‑azachromanones via Horner-Wadsworth_Emmons. Chem. Het. Compd. 2020. V.56, № 2. P. 213-218. https://doi.org/10.1007/s10593-020-02646-z
41. Abdurakhmanova E.R., Brusnakov M.Y., Golovchenko O.V., Pilyo S.G., Velychko N.V., Hariden E.A., Prichard M.N., James S.H., Zhirnov V.V., Brovarets V.S. Synthesis and in vitro anticitomegalovirus activity of 5-hydroxyalkylamino-1,3-oxazoles derivatives. Med. Chem. Res., 2020. V.29, № 9. P. 1669-1675. https://doi.org/10.1007/s00044-020-02593-6
42. L.M. Potikha, V.S. Brovarets. Synthesis of new antineoplastic agents based on imadazo[2,1-a]pyridine. Chem. Heterocycl. Compd. 2020. V.56, № 11. P. 1460-1464. https://doi.org/10.1007/s10593-020-02838-7
43. Potikha L.M., Brovarets V.S.. Synthethis of imidazole[2,1-b][1,3]thiazoles – potential anticancer agents derived from p-bromodipnones. Chem. Heterocycl. Compd. 2020. V.56, № 8. P. 1073-1077. http://dx.doi.org/10.1007/s10593-020-02776-4
44. Grygorenko O.O., Moskvina V.S., Hryshchuk O.V., Tymtsunik A.V. Cycloadditions of alkenylboronic derivatives. Synthesis. 2020. V. 52. P. 2761-2780. http://dx.doi.org/10.1055/s-0040-1707159

45. Grygorenko O.O., Hutskalova V., Moskvina V.S. Bicyclic 6-6 systems with one bridgehead (ring junction) nitrogen atom: three extra heteroatoms (2:1). Chapter in collection: “Reference module in chemistry, molecular sciences and chemical engineering”. 2020. http://dx.doi.org/10.1016/B978-0-12-409547-2.14958-3
46. Nizhenkovska I.V., Matskevych K.V., Golovchenko O.V., Golovchenko O.I.. Synthesis and pharmacological trials of new phosphorylated oxazole derivatives antihypertensive properties. Maced. Pharm. Bull. 2020. V.66, № 1. P. 51-52. http://dx.doi.org/10.33320/maced.pharm.bull.2020.66.03.025
47. Velihina Ye., Scattolin T., Bondar D., Pil’o S.,Obernikhina N.,Kachkovskyi O.,Semenyuta I., Caligiuri I., Rizzolio F., Brovarets V.,Karpichev Ye., Nolan S.P. Synthesis, in silico and in vitro evaluation of novel oxazolopyrimidines as promising anticancer agents. Helv. Chim. Acta. 2020. P. 1‑12. https://doi.org/10.1002/hlca.202000169
48. Obernikhina N., Pavlenko O., Kachkovsky A., Brovarets V.. Quantum-chemical and experimental estimation of non-bonding level (Fermi level) and π‑electron afinity of conjugated systems. Polycycl. Arom. Comp. 2020. P. 1-10. https://doi.org/10.1080/10406638.2019.1710855
49. Blyuss K.B., Al Basir F., Tsygankova V.A. Control of mosaic disease using microbial biostimulants: insights from mathematical modelling. Ricerche Mat. 2020. V.69. P. 437-455. https://doi.org/10.1007/s11587-020-00508-6
50. Davydenko I.G., Slominskiy Yu.L., Obernikhina N.V., Kachkovsky A.D., Tolmachev A.. Infrared Polyene Radical-Cation Derived from 7,8-Dihydrobenzo[c,d]Furo[2,3-f]Indole: Synthesis, Spectra and Nature of Electron Transitions. Chemistry Select. 2020. V.5. P. 674-681. https://doi.org/10.1002/slct.201904086

51. Maiko K.O., Dmitruk I. M., Obernikhina N.V., Kachkovsky A. D. Solitonic‑like excitations in cations of linear conjugated systems. Monatsh. Chem. 2020. V.151, № 4. P. 559-566. https://doi.org/10.1007/s00706-020-02572-y
1. Pluhařová K., Leontovyčová H., Stoudková V., Pospíchalová R., Maršík P., Klouček P., Starodubtseva A., Iakovenko O., Krčková Z., Valentová O., Burketová L., Janda M., Kalachova T. “Salicylic Acid Mutant Collection” as a Tool to Explore the Role of Salicylic Acid in Regulation of Plant Growth under a Changing Environment. International journal of molecular sciences. 2019. Vol. 20 (24). P. 6365. DOI: 10.3390/ijms20246365.
https://www.mdpi.com/1422-0067/20/24/6365
2. Xie Y., Kril L.M., Yu T., Zhang W., Frasinyuk M.S., Bondarenko S.P., Kondratyuk K.M., Hausman E., Martin Z.M., Wyrebek P.P., Liu X., Deaciuc A., Dwoskin L.P., Chen J., Zhu H., Zhan C.-G., Sviripa V.M., Blackburn J., Watt D.S., Liu C. Semisynthetic aurones inhibit tubulin polymerization at the colchicine-binding site and repress PC-3 tumor xenografts in nude mice and myc-induced T-ALL in zebrafish. Scientific Reports. 2019. Vol. 9 (1). P. 6439. DOI: 10.1038/s41598-019-42917-0.
https://www.nature.com/articles/s41598-019-42917-0
3. Kachaeva M.V., Hodyna D.M., Obernikhina N.V., Pilyo S.G., Kovalenko Y.S., Prokopenko V.M., Kachkovsky O.D., Brovarets V.S. Dependence of the anticancer activity of 1,3-oxazole derivatives on the donor/acceptor nature of his substitues. Journal of Heterocyclic Chemistry. 2019. Vol. 56 (11). P. 3122-3134. DOI: 10.1002/jhet.3711.
https://onlinelibrary.wiley.com/doi/abs/10.1002/jhet.3711
4. Shaydyuk Y.O., Boyko O.P., Kachkovsky O.D., Slominsky Y.L., Bricks J.L., Belfield K.D., Bondar M.V. Electronic nature of new styryl dye bases: Linear photophysical, photochemical, and transient absorption spectroscopy studies. Dyes and Pigments. 2019. Vol. 170. DOI: 10.1016/j.dyepig.2019.107582.
https://www.sciencedirect.com/science/article/abs/pii/S0143720819306011
5. Kalachova T., Leontovyčová H., Iakovenko O., Pospíchalová R., Maršík P., Klouček P., Janda M., Valentová O., Kocourková D., Martinec J., Burketová L., Ruelland E. Interplay between phosphoinositides and actin cytoskeleton in the regulation of immunity related responses in Arabidopsis thaliana seedlings. Environmental and Experimental Botany. 2019. Vol. 167. DOI: 10.1016/j.envexpbot.2019.103867.
https://www.sciencedirect.com/science/article/abs/pii/S009884721930454X
6. Mrug G.P., Biletska I.M., Bondarenko S.P. Sviripa V.M., Frasinyuk M.S. Trifluoroacetylation of 2-Methyl- and 2-Ethylchromones: A Convenient Access to 2-Trifluoroacetonyl Chromones. ChemistrySelect. 2019. Vol. 4 (39). P. 11506-11510. DOI: 10.1002/slct.201903629.
https://onlinelibrary.wiley.com/doi/abs/10.1002/slct.201903629

7. Kovalchuk V., Voronkina A, Binnewerg B., Schubert M., Muzychka L., Wysokowski M., Tsurkan M.V., Bechmann N., Petrenko I., Fursov A., Martinovic R., Ivanenko V.N., Fromont J., Smolii O.B., Joseph Y., Giovine M., Erpenbeck D., Gelinsky M., Springer A., Guan K., Bornstein S.R., Ehrlich H. Naturally drug-loaded chitin: Isolation and applications. Marine Drugs. 2019. Vol. 17 (10). Р. 574. DOI: 10.3390/md17100574.
https://www.mdpi.com/1660-3397/17/10/574
8. Kartsev V., Geronikaki A., Bua S., Nocentini A, Petrou A., Lichitsky B., Frasinyuk M., Leitans J., Kazaks A., Tars K., Supuran C.T. Extending the inhibition profiles of coumarin-based compounds against human carbonic anhydrases: Synthesis, biological, and in silico evaluation. Molecules. 2019. Vol. 24 (19). Р. 3580. DOI: 10.3390/molecules24193580. https://doi.org/10.3390/molecules24193580
9. Schubert M., Binnewerg B., Voronkina A., Muzychka L., Wysokowski M., Petrenko I., Kovalchuk V., Tsurkan M., Martinovic R., Bechmann N., Ivanenko V.N., Fursov A., Smolii O.B., Fromont J., Joseph Y., Bornstein S.R., Giovine M., Erpenbeck D., Guan K., Ehrlich H. Naturally prefabricated marine biomaterials: Isolation and applications of flat chitinous 3D scaffolds from Ianthella labyrinthus (demospongiae: Verongiida). International Journal of Molecular Sciences. 2019. Vol.20 (20). Р. 5105. DOI: 10.3390/ijms20205105. https://doi.org/10.3390/ijms20205105
10. Kornii Y., Chumachenko S., Shablykin O., Prichard M.N., James S.H., Hartline C., Zhirnov V., Brovarets V. New 2-Oxoimidazolidine Derivatives: Design, Synthesis and Evaluation of Anti-BK Virus Activities in Vitro. Chemistry and Biodiversity. 2019. Vol. 16 (10). e1900391. DOI: 10.1002/cbdv.201900391. https://doi.org/10.1002/cbdv.201900391
11. Savych O., Kuchkovska Y.O., Bogolyubsky A.V., Konovets A.I., Gubina K.E., Pipko S.E., Zhemera A.V., Grishchenko A.V., Khomenko D.N., Brovarets V.S., Doroschuk R., Moroz Y.S., Grygorenko O.O. One-Pot Parallel Synthesis of 5-(Dialkylamino)tetrazoles. ACS Combinatorial Science. 2019. Vol. 21 (9). P. 635-642. https://doi.org/10.1021/acscombsci.9b00120
12. Garazd M.M., Frasinyuk M.S. Synthesis of Isoflavone–Amino-Acid Conjugates. Chemistry of Natural Compounds. 2019. Vol. 55 (5). P. 813-817. DOI: 10.1007/s10600-019-02821-5.
https://link.springer.com/article/10.1007/s10600-019-02821-5
13. Pokotylo I., Kravets V., Ruelland E. Salicylic acid binding proteins (SABPs): The hidden forefront of salicylic acid signalling. International Journal of Molecular Sciences. 2019. Vol. 20 (18). Р. 4377. DOI: 10.3390/ijms20184377.
https://www.mdpi.com/1422-0067/20/18/4377
14. Lutsyk P., Piryatinski Y., Shandura M., Alaraimi M., Tesa M., Arnaoutakis G.E., Melvin A.A., Kachkovsky O., Verbitsky A., Rozhin A. Self-Assembly for Two Types of J-Aggregates: Cis-Isomers of Dye on the Carbon Nanotube Surface and Free Aggregates of Dye trans-Isomers. Journal of Physical Chemistry C. 2019. Vol. 123 (32). P. 19903-19911. DOI: 10.1021/acs.jpcc.9b03341.
https://pubs.acs.org/doi/10.1021/acs.jpcc.9b03341

15. Kachaeva M.V., Pilyo S.G., Hartline C.B., Harden E.A., Prichard M.N., Zhirnov V.V., Brovarets V.S. In vitro activity of novel derivatives of 1,3-oxazole-4-carboxylate and 1,3-oxazole-4-carbonitrile against human cytomegalovirus. Medicinal Chemistry Research. 2019. Vol. 28 (8). Р. 1205-1211. DOI: 10.1007/s00044-019-02365-x.
https://link.springer.com/article/10.1007/s00044-019-02365-x
16. Kondratyuk K.M., Dluzhevskii V.A., Bondarenko S.P., Brovarets V.S., Frasinyuk M.S. Synthesis of Coumarin-4-ylmethyl Phosphonic Acids. Chemistry of Natural Compounds. 2019. Vol. 55 (4). Р. 632-637. DOI: 10.1007/s10600-019-02766-9.
https://link.springer.com/article/10.1007/s10600-019-02766-9
17. Bondarenko S.P., Mrug G.P., Vinogradova V.I., Khilya V.P., Frasinyuk M.S. Conjugation of the Alkaloid Anabasine to Coumarins. Chemistry of Natural Compounds. 2019. Vol. 55 (4). Р. 628-631. DOI: 10.1007/s10600-019-02765-w.
https://link.springer.com/article/10.1007/s10600-019-02765-w
18. Chernykh A.V., Melnykov K.P., Tolmacheva N.A., Kondratov I.S., Radchenko D.S., Daniliuc C.G., Volochnyuk D.M., Ryabukhin S.V., Kuchkovska Y.O., Grygorenko O.O. Last of the gem-Difluorocycloalkanes: Synthesis and Characterization of 2,2-Difluorocyclobutyl-Substituted Building Blocks. Journal of Organic Chemistry. 2019. Vol. 84 (13). Р. 8487-8496. DOI: 10.1021/acs.joc.9b00719.
https://pubs.acs.org/doi/10.1021/acs.joc.9b00719

19. Patrylak L.K., Okhrimenko M.V., Levterov A.M., Konovalov S.V., Yakovenko A.V., Zubenko S.O. Engine performance and emission of biodiesel fuel prepared from different Ukrainian natural oils. Chemical Papers. 2019. Vol. 73 (7). Р. 1823-1832. DOI: 10.1007/s11696-019-00755-4.
https://link.springer.com/article/10.1007/s11696-019-00755-4
20. Derevyanchuk M., Kretynin S., Kolesnikov Y., Litvinovskaya R., Martinec J., Khripach V., Kravets V. Seed germination, respiratory processes and phosphatidic acid accumulation in Arabidopsis diacylglycerol kinase knockouts – The effect of brassinosteroid, brassinazole and salinity. Steroids. 2019. Vol. 147. Р. 28-36. DOI: 10.1016/j.steroids.2019.04.002. https://doi.org/10.1016/j.steroids.2019.04.002
21. Stepaniuk O.O., Rudenko T.V., Vashchenko B.V., Matvienko V.O., Kondratov I.S., Tolmachev A.A., Grygorenko O.O. Reaction of β-alkoxyvinyl α-ketoesters with acyclic NCN binucleophiles – Scalable approach to novel functionalized pyrimidines. Tetrahedron. 2019. Vol. 75 (25). Р. 3472-3478. DOI: 10.1016/j.tet.2019.05.005.
https://www.sciencedirect.com/science/article/abs/pii/S0040402019305125

22. Patrylak L.K., Krylova M.M., Pertko O.P., Voloshyna Y.G. Linear hexane isomerization over Ni-containing pentasils. Journal of Porous Materials. 2019. Vol. 26 (3). Р. 861-868. DOI: 10.1007/s10934-018-0685-1.
https://link.springer.com/article/10.1007/s10934-018-0685-1
23. Mrug G.P., Myshko N.V., Bondarenko S.P., Sviripa V.M., Frasinyuk M.S. One-Pot Synthesis of B-Ring Ortho-Hydroxylated Sappanin-Type Homoisoflavonoids. Journal of Organic Chemistry. 2019. Vol. 84 (11). Р. 7138-7147. https://doi.org/10.1021/acs.joc.9b00814

24. Gerus I.I., Zhuk Y.I., Tarasenko K.V., Shaitanova E.N., Sorochinskii A.E. Synthesis and evaluation of new tri- and difluoromethyl containing 2,6,9-trioxabicyclo[3.3.1]nonanes. Journal of Fluorine Chemistry. 2019. Vol. 222-223. Р. 100-105. DOI: 10.1016/j.jfluchem.2019.04.017.
https://www.sciencedirect.com/science/article/abs/pii/S0022113919300594

25. Kolodiazhnyi O.I. Stereochemistry of electrophilic and nucleophilic substitution at phosphorus. Phosphorus, Sulfur and Silicon and the Related Elements. 2019. Vol. 194 (4-6). Р. 396-400. DOI: 10.1080/10426507.2018.1521409.
https://www.tandfonline.com/doi/abs/10.1080/10426507.2018.1521409?journalCode=gpss20

26. Kolodiazhna A., Kolodiazhnyi O. Stereoselective syntheses of sanshool derivatives. Phosphorus, Sulfur and Silicon and the Related Elements. 2019. Vol. 194 (4-6). Р. 275-276. DOI: 10.1080/10426507.2018.1514404.
https://www.tandfonline.com/doi/abs/10.1080/10426507.2018.1514404?journalCode=gpss20

27. Muzychka L.V., Yaremchuk I.O., Verves E.V., Smolii O.B. Pyrrolo[2,3-d]pyrimidine derivatives in the synthesis of a novel heterocyclic system 2a,5a,7-triazaacenaphthylene. Chemistry of Heterocyclic Compounds. 2019. Vol. 55 (4-5). Р. 397-400. DOI: 10.1007/s10593-019-02471-z.
http://hgs.osi.lv/index.php/hgs/article/view/5009
28. Popova A.V., Bondarenko S.P., Frasinyuk M.S. Aurones: Synthesis and Properties. Chemistry of Heterocyclic Compounds. 2019. Vol. 55 (4-5). Р. 285-299. DOI: 10.1007/s10593-019-02457-x.
https://link.springer.com/article/10.1007/s10593-019-02457-x
29. Moskvina V.S., Khilya V.P. Aryl alkynoates in the radical synthesis of coumarins. Chemistry of Heterocyclic Compounds. 2019. Vol. 55 (4-5). Р. 300-306. DOI: 10.1007/s10593-019-02458-w.
https://link.springer.com/article/10.1007/s10593-019-02458-w
30. Mrug G.P., Demidchuk B.A., Bondarenko S.P., Gorbulenko N.V., Frasinyuk M.S. Synthesis and Properties of 2-Carboxyethyland 2-Carboxypropylchromones. Chemistry of Natural Compounds. 2019. Vol. 55 (3). Р. 443-448. DOI: 10.1007/s10600-019-02710-x.
https://link.springer.com/article/10.1007/s10600-019-02710-x
31. Kachaeva M.V., Obernikhina N.V., Veligina E.S., Zhuravlova M.Y., Prostota Y.O., Kachkovsky O.D., Brovarets V.S. Estimation of biological affinity of nitrogen-containing conjugated heterocyclic pharmacophores. Chemistry of Heterocyclic Compounds. 2019. Vol. 55 (4-5). Р. 448-454. DOI: 10.1007/s10593-019-02478-6.
https://link.springer.com/article/10.1007/s10593-019-02478-6
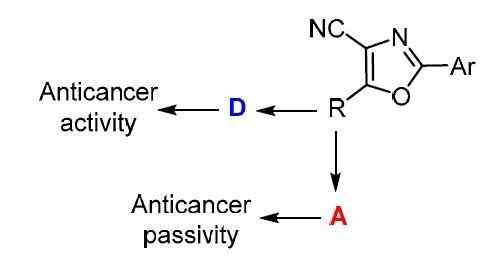
32. Solomyannyi R.N., Shablykina O.V., Moskvina V.S., Khilya V.P., Rusanov E.B., Brovarets V.S. 8-(Methyl(phenyl)sulfonyl)-2,6-dihydroimidazo[1,2-c]-pyrimidin-5(3Н)-ones and 9-(methyl(phenyl)sulfonyl)-2,3,4,7-dihydro-6H-pyrimido[1,6-a]pyrimidin-6-ones: synthesis and antiviral activity. Chemistry of Heterocyclic Compounds. 2019. Vol. 55 (4-5). Р. 401-407. DOI: 10.1007/s10593-019-02472-y.
http://hgs.osi.lv/index.php/hgs/article/view/5015
33. Fainleib A., Grigoryeva O., Vashchuk А., Starostenko O., Rogalsky S., Rios de Anda A., Nguyen T.-T.-T., Grande D. Effect of ionic liquids on kinetic parameters of dicyanate ester polycyclotrimerization and on thermal and viscoelastic properties of resulting cyanate ester resins. Express Polymer Letters. 2019. Vol. 13 (5). Р. 469-483. http://dx.doi.org/10.3144/expresspolymlett.2019.39
34. Noskov Y., Sorochinsky A., Kukhar V., Pud A. Polyaniline Doping by α,α-Difluoro-β-amino Acids. ACS Omega. 2019. Vol. 4 (4). Р. 7400-7410. https://doi.org/10.1021/acsomega.9b00207

35. Yagupolskii Y.L., Pavlenko N.V., Gerus I.I., Peng S., Nappa M. 1,1,1,4,4,4-Hexafluorobut-2-yne (HFB) as a Versatile Dipolarophile for [3+2] Cycloaddition with 1,3-Dipoles toward Azoles with Adjacent CF 3 Groups. ChemistrySelect. 2019. Vol. 4 (15). Р. 4604-4610. DOI: 10.1002/slct.201900387.
https://onlinelibrary.wiley.com/doi/abs/10.1002/slct.201900387

36. Blyuss K.B., Fatehi F., Tsygankova V.A., Biliavska L.O., Iutynska G.O., Yemets A.I., Blume Y.B. RNAi-based biocontrol of wheat nematodes using natural poly-component biostimulants. Frontiers in Plant Science. 2019. Vol. 10. Стаття № 483. https://doi.org/10.3389/fpls.2019.00483
37. Semenyuta I.V., Kobzar O.L., Hodyna D.M., Brovarets V.S., Metelytsia L.O. In silico study of 4-phosphorylated derivatives of 1,3-oxazole as inhibitors of Candida albicans fructose-1,6-bisphosphate aldolase II. Heliyon. 2019. Vol. 5 (4). Стаття № e01462. https://doi.org/10.1016/j.heliyon.2019.e01462
38. Little I., Alorkpa E., Khan V., Povazhnyi V., Vasiliev A. Efficient porous adsorbent for removal of cesium from contaminated water. Journal of Porous Materials. 2019. Vol. 26 (2). Р. 361-369. DOI: 10.1007/s10934-018-0608-1.
https://link.springer.com/article/10.1007/s10934-018-0608-1
39. Shchodryi V., Obernikhina N., Shaydyk Y., Kachkovsky O., Yu S., Tkachuk Z. Fluorescent Probe for Investigation of Influence of Ribonucleosides with D-Mannitol. 2019 IEEE 39th International Conference on Electronics and Nanotechnology, ELNANO. 2019. Р. 385-389. Стаття № 8783955. DOI: 10.1109/ELNANO.2019.8783955.
https://ieeexplore.ieee.org/abstract/document/8783955
40. Buldenko V.M., Trush V.V., Kobzar O.L., Drapailo A.B., Kalchenko V.I., Vovk A.I. Calixarene-based phosphinic acids as inhibitors of protein tyrosine phosphatases. Bioorganic and Medicinal Chemistry Letters. 2019. Vol. 29 (6). Р. 797-801. DOI: 10.1016/j.bmcl.2019.01.026.
https://www.sciencedirect.com/science/article/pii/S0960894X19300393

41. Bondarenko S.P., Frasinyuk M.S. Chromone Alkaloids: Structural Features, Distribution in Nature, and Biological Activity. Chemistry of Natural Compounds. 2019. Vol. 55 (2). Р. 201-234. DOI: 10.1007/s10600-019-02656-0.
https://link.springer.com/article/10.1007/s10600-019-02656-0
42. Popova A.V., Bondarenko S.P., Vinogradova V.I., Frasinyuk M.S. Synthesis of anabasine-containing aminomethyl derivatives of 6-hydroxyaurones. Chemistry of Heterocyclic Compounds. 2019. Vol. 55 (3). Р. 212-216. DOI: 10.1007/s10593-019-02444-2.
https://link.springer.com/article/10.1007/s10593-019-02444-2
43. Hamouda Z., Wojkiewicz J.-L., Pud A.A., Kone L., Bergheul S., Lasri T. Development of a Dual-band Printed Antenna Based on a Nanocomposite Material made of Polyaniline charged by Iron-Nickel Nanopowder. 13th European Conference on Antennas and Propagation. EuCAP 2019. Vol. 2019. Стаття № 8740128.
https://ieeexplore.ieee.org/document/8740128
44. Casciuc I., Horvath D., Gryniukova A., Tolmachova K.A., Vasylchenko O.V., Borysko P., Moroz Y.S., Bajorath J., Varnek A. Pros and cons of virtual screening based on public “Big Data”: In silico mining for new bromodomain inhibitors. European Journal of Medicinal Chemistry. 2019. Vol. 165. Р. 258-272. DOI: 10.1016/j.ejmech.2019.01.010.
https://www.sciencedirect.com/science/article/pii/S0223523419300169

45. Klinger C., Zółtowska-Aksamitowska S.Z., Wysokowski M., Tsurkan M.V., Galli R., Petrenko I., Machałowski T., Ereskovsky A., Martinović R., Muzychka L., Smolii O.B., Bechmann N., Ivanenko V., Schupp P.J., Jesionowski T., Giovine M., Joseph Y., Bornstein S.R., Voronkina A., Ehrlich H. Express method for isolation of ready-to-use 3D chitin scaffolds from aplysina archeri(aplysineidae: verongiida) demosponge. Marine Drugs. 2019. Vol. 17 (2). Стаття № 131. https://doi.org/10.3390/md17020131
46. Trush M., Metelytsia L., Semenyuta I., Kalashnikova L., Papeykin O., Venger I., Tarasyuk O., Bodachivska L., Blagodatnyi V., Rogalsky S. Reduced ecotoxicity and improved biodegradability of cationic biocides based on ester-functionalized pyridinium ionic liquids. Environmental Science and Pollution Research. 2019. Vol. 26 (5). Р. 4878-4889. DOI: 10.1007/s11356-018-3924-8.
https://link.springer.com/article/10.1007/s11356-018-3924-8
47. Feskov I.O., Chernykh A.V., Kuchkovska Y.O., Daniliuc C.G., Kondratov I.S., Grygorenko O.O. 3-((Hetera)cyclobutyl)azetidines, “stretched” Analogues of Piperidine, Piperazine, and Morpholine: Advanced Building Blocks for Drug Discovery. Journal of Organic Chemistry. 2019. Vol. 84 (3). Р. 1363-1371. DOI: 10.1021/acs.joc.8b02822.
https://pubs.acs.org/doi/10.1021/acs.joc.8b02822

48. Volochnyuk D.M., Ryabukhin S.V., Moroz Y.S., Savych O., Chuprina A., Horvath D., Zabolotna Y., Varnek A., Judd D.B. Evolution of commercially available compounds for HTS. Drug Discovery Today. 2019. Vol. 24 (2). Р. 390-402. DOI: 10.1016/j.drudis.2018.10.016.
https://www.sciencedirect.com/science/article/pii/S1359644618302423
49. Moshynets O.V., Babenko L.M., Rogalsky S.P., Iungin O.S., Foster J., Kosakivska I.V., Potters G., Spiers A.J. Priming winter wheat seeds with the bacterial quorum sensing signal N-hexanoyl-L-homoserine lactone (C6-HSL) shows potential to improve plant growth and seed yield. PLoS ONE. 2019. Vol. 14 (2). Стаття № e0209460. https://doi.org/10.1371/journal.pone.0209460
50. Shaala L.A., Asfour H.Z., Youssef D.T.A., Zółtowska-Aksamitowska S.Z., Wysokowski M., Tsurkan M., Galli R., Meissner H., Petrenko I., Tabachnick K., Ivanenko V.N., Bechmann N., Muzychka L.V., Smolii O.B., Martinović R., Joseph Y., Jesionowski T., Ehrlich H. New source of 3D chitin scaffolds: The red sea demosponge pseudoceratina arabica(pseudoceratinidae, verongiida). Marine Drugs. 2019. Vol. 17 (2). Стаття № 92. https://doi.org/10.3390/md17020092
51. Kolotylo M., Holovatiuk V., Bondareva J., Lukin O., Rozhkov V. Synthesis of sulfonimide-based dendrimers and dendrons possessing mixed 1 → 2 and 1 → 4 branching motifs. Tetrahedron Letters. 2019. Vol. 60 (4). Р. 352-354. DOI: 10.1016/j.tetlet.2018.12.052.
https://www.sciencedirect.com/science/article/pii/S004040391831503X

52. Voloshyna Y.G., Pertko O.P., Patrylak L.K. Effect of the Method of Modification of Zeolite X on Selectivity of Catalytic Methylation of Toluene. Theoretical and Experimental Chemistry. 2019. Vol. 54 (6). Р. 395-400. DOI: 10.1007/s11237-019-09586-6.
https://link.springer.com/article/10.1007/s11237-019-09586-6
53. Kachaeva M.V., Pilyo S.G., Zhirnov V.V., Brovarets V.S. Synthesis, characterization, and in vitro anticancer evaluation of 2-substituted 5-arylsulfonyl-1,3-oxazole-4-carbonitriles. Medicinal Chemistry Research. 2019. Vol. 28 (1). Р. 71-80. DOI: 10.1007/s00044-018-2265-y.
https://link.springer.com/article/10.1007/s00044-018-2265-y
54. Moshynets O., Bardeau J.-F., Tarasyuk O., Makhno S., Cherniavska T., Dzhuzha O., Potters G., Rogalsky S. Antibiofilm activity of polyamide 11 modified with thermally stable polymeric biocide polyhexamethylene guanidine 2-naphtalenesulfonate. International Journal of Molecular Sciences. 2019. Vol. 20 (2). Стаття № 348. https://doi.org/10.3390/ijms20020348
55. Protasov A.A., Morozovskaya I.A., Laskovenko N.N., Rogalskiy S.P. Composition and structure of zooperiphyton on the experimental substrates in the Kaniv reservoir. The many-year aspect. Hydrobiological Journal. 2019. Vol. 55 (5). Р. 3-19. DOI: 10.1615/HydrobJ.v55.i5.10.
https://www.semanticscholar.org/paper/Composition-and-Structure-of-Zooperiphyton-on-the-Protasov-Morozovskaya/e1ee8434648e0c41b134cbc2d82a59e2c56af051
56. Romanenko V.D. α-Heteroatom-substituted gem-bisphosphonates: Advances in the synthesis and prospects for biomedical application. Current Organic Chemistry. 2019. Vol. 23 (5). Р. 530-615. DOI: 10.2174/1385272823666190401141844.
http://www.eurekaselect.com/node/171178/article/-heteroatom-substituted-gem-bisphosphonates-advances-in-the-synthesis-and-prospects-for-biomedical-application

57. Trush M.M., Kovalishyn V., Ocheretniuk A.D., Kobzar O.L., Kachaeva M.V., Brovarets V.S., Metelytsia L.O. QSAR study of some 1,3-oxazolylphosphonium derivatives as new potent anti-Candida agents and their toxicity evaluation. Current Drug Discovery Technologies. 2019. Vol. 16 (2). Р. 204-209. https://doi.org/10.2174/1570163815666180418145422
58. Kolodiazhnyi O.I. Stereochemistry of electrophilic and nucleophilic substitutions at phosphorus. Pure and Applied Chemistry. 2019. Vol. 91 (1). Р. 43-57. DOI: 10.1515/pac-2018-0807.
https://www.degruyter.com/view/j/pac.2019.91.issue-1/pac-2018-0807/pac-2018-0807.xml
59. Pavliuk A.V., Bezugly Y.V., Sukhoveev V.V., Kashkovsky V.I. A convenient method for efficient synthesis of isoxazole-containing thiadiazepine derivatives. Chemistry of Heterocyclic Compounds. 2019. Vol. 55. Р. 1274-1277. DOI: 10.1007/s10593-019-02612-4.
https://link.springer.com/article/10.1007/s10593-019-02612-4
60. Pud A.A., Ogurtsov N.A., Noskov Y.V., Mikhaylov S.D., Piryatinski Y.P., Bliznyuk V.N. On the importance of interface interactions in core-shell nanocomposites of intrinsically conducting polymers. Semiconductor Physics, Quantum Electronics and Optoelectronics. 2019. Vol. 22 (4). Р. 470-478. DOI: 10.15407/spqeo22.04.470.
https://ouci.dntb.gov.ua/en/works/lxGk8vG4/
61. Malets Y.S., Moskvina V.S., Grygorenko O.O., Brovarets V.S. Synthesis of azachromones and azachromanones. Chemistry of Heterocyclic Compounds. 2019. Vol. 55(11). Р. 1007-1012. DOI: 10.1007/s10593-019-02570-x.
https://link.springer.com/article/10.1007/s10593-019-02570-x
62. Solomyannyi R., Slivchuk S., Smee D., Choi J.-A., Rusanov E., Zhirnov V., Brovarets V. In vitro activity of the novel pyrimidines and their condensed derivatives against poliovirus. Current Bioactive Compounds. 2019. Vol. 15 (5). Р. 582-591. DOI: 10.2174/1573407214666180720120509.
https://www.eurekaselect.com/node/163944/article/in-vitro-activity-of-the-novel-pyrimidines-and-their-condensed-derivatives-against-poliovirus

63. Pavlenko O.L., Dmytrenko O.P., Kulish M.P., Sendiuk V.A., Obernikhina N.V., Prostota Y.O., Kachkovsky O.D., Bulavin L.A. Electron structure and optical properties of conjugated systems in solutions. Springer Proceedings in Physics. 2019. Vol. 223. Р. 225-248. DOI: 10.1007/978-3-030-21755-6_9.
https://link.springer.com/chapter/10.1007/978-3-030-21755-6_9
64. Bliznyuk V.N., Kołacińska K., Pud A.A., Ogurtsov N.A., Noskov Y.V., Powell B.A., Devol T.A. High effectiveness of pure polydopamine in extraction of uranium and plutonium from groundwater. RSC Advances. 2019. Vol. 9 (52). Р. 30052-30063. DOI: 10.1039/c9ra06392g.
https://pubs.rsc.org/en/content/articlelanding/2019/ra/c9ra06392g#!divAbstract
65. Shablykin O.V., Kornii Y.E., Dyakonenko V.V., Shablykina O.V., Brovarets V.S. Synthesis and anticancer activity of new substituted imidazolidinone sulfonamides. Current Chemistry Letters. 2019. Vol. 8 (4). Р. 199-210. DOI: 10.5267/j.ccl.2019.005.003.
http://growingscience.com/beta/ccl/3252-synthesis-and-anticancer-activity-of-new-substituted-imidazolidinone-sulfonamides.html
66. Tsygankova V.A., Andrusevich Y.V., Shysha E.N., Biliavska L.O., Galagan T.O., Galkin A.P., Yemets A.I., Iutynska G.A., Blume Y.B. RNAi-mediated resistance against plant parasitic nematodes of wheat plants obtained in vitro using bioregulators of microbiological origin. Current Chemical Biology. 2019. Vol. 13 (1). Р. 73-89. DOI: 10.2174/2212796812666180507130017.
https://www.eurekaselect.com/node/161879/article/rnai-mediated-resistance-against-plant-parasitic-nematodes-of-wheat-plants-obtained-in-vitro-using-bioregulators-of-microbiological-origin

67. Kachkovsky A.D., Pavlenko E.L., Sheludko E.V., Kulish N.P., Dmitrenko O.P., Sendyuk V.A., Smertenko P.S., Kremenitsky V.V., Tarasyuk O.P., Rogalsky S.P. Composite ‘graphene nanoplatelets – fluorine-containing polyamide’: Synthesis, properties and quantum-chemical simulation of electroconductivity. Functional Materials. 2019. Vol. 26 (1). Р. 100-106. DOI: 10.15407/FM26.01.100.
http://dspace.nbuv.gov.ua/handle/123456789/157405
68. Obernikhina N., Kachaeva M., Shchodryi V., Prostota Y., Kachkovsky O., Brovarets V., Tkachuk Z. Topological Index of Conjugated Heterocyclic Compounds as Their Donor/Acceptor Parameter. Polycyclic Aromatic Compounds. 2019. DOI: 10.1080/10406638.2018.1538056.
https://www.tandfonline.com/doi/abs/10.1080/10406638.2018.1538056?journalCode=gpol20
69. Bugera M., Trofymchuk S., Tarasenko K., Zaporozhets O., Pustovit Y., Mykhailiuk P.K. Deoxofluorination of Aliphatic Carboxylic Acids: A Route to Trifluoromethyl-Substituted Derivatives. Journal of Organic Chemistry. 2019. Vol. 84 (24). Р. 16105-16115. DOI: 10.1021/acs.joc.9b02596.
https://pubs.acs.org/doi/abs/10.1021/acs.joc.9b02596

70. Obernikhina N., Zhuravlova M., Kachkovsky O., Kobzar O., Brovarets V., Pavlenko O., Kulish M., Dmytrenko O. Stability of fullerene complexes with oxazoles as biologically active compounds. Applied Nanoscience (Switzerland). 2019. DOI: 10.1007/s13204-019-01225-9.
https://link.springer.com/article/10.1007/s13204-019-01225-9
71. Kretynin S.V., Kolesnikov Y.S., Derevyanchuk M.V., Kalachova T.A., Blume Y.B., Khripach V.A., Kravets V.S. Brassinosteroids application induces phosphatidic acid production and modify antioxidant enzymes activity in tobacco in calcium-dependent manner. Steroids. 2019. Стаття № 108444. https://doi.org/10.1016/j.steroids.2019.108444
72. Pavluik O., Bezugly Y., Kashkovsky V. Ring closing metathesis strategies to isoxazole containing thiadiazepines. French-Ukrainian Journal of Chemistry. 2019. Vol. 7 (1). P. 104-112.
https://doi.org/10.17721/fujcV7I1P104-112
73. Tsygankova V.A., Andrusevich Ya.V., Shtompel O.I., Kopich V.M., Solomyanny R.M., Brovarets V.S. Study of regulating activity of synthetic low molecular weight heterocyclic compounds, derivatives of pyrimidine on growth of tomato (Solanum lycopersicum L.) seedlings. International Journal of ChemTech Research, 2019, Vol.12 No.05, pp 26-38. http://dx.doi.org/10.20902/IJCTR.2019.120504
74. Tsygankova V.A., Andrusevich Ya.V, Shtompel O.I, Kopich V.M, Panchyshyn S.Ya, Vydzhak R.M, Brovarets V.S (2019). Application of Pyrazole Derivatives As New Substitutes of Auxin IAA To Regulate Morphometric and Biochemical Parameters of Wheat (Triticum Aestivum L.) Seedlings. JOURNAL OF ADVANCES IN AGRICULTURE, 10, 1772-1786. https://doi.org/10.24297/jaa.v10i0.8341
75. Adekunle Odunayo Adejuwon, Victoria Anatolyivna Tsygankova, Abiola Muhammad Adeosun, Adefemi Olawale Falase, Olubunmi Sharon Obayemi, Fatai Ishola Amusa. Phytochemical screening and antimicrobial efficacy of the root bark of Securidaca longipedunculata extracts. AMERICAN JOURNAL OF RESEARCH IN MEDICAL SCIENCES. 2019. Vol. 5, No 1, P. 7 – 13. http://dx.doi.org/10.5455/ajrms.20181204113428
76. Adejuwon A.O. and Tsygankova V.A. Phyto-Chemical Screening and Ethno-Botanical Properties of Selected Plants of the Obafemi Awolowo University, Ile-Ife, Nigeria. J Complement Med Alt Healthcare. 2019; 9(3): 555761. http://dx.doi.org/10.19080/JCMAH.2019.09.555761
77. Adekunle Odunayo Adejuwon, Victoria Anatolyivna Tsygankova, Olubunmi Sharon Obayemi. ?-Amylase Production Using Aspergilus vadensis Isolated From Pulverized Cocoa Seeds. Life Science Journal 2019; 16(8). P. 64 – 70. http://dx.doi.org/10.7537/marslsj160819.08
78. Victoria Tsygankova et al. (2019), Screening of New Effective Regulators of Oilseed Rape Growth Among Derivatives of Oxazole and Oxazolopyrimidine. Proceedings of World Congress and Expo on Chemistry during November 15-17, 2018 in Rome, Italy. Int J Pharm Sci & Scient Res. 5:3, 34. https://www.researchgate.net/publication/333017109_Screening_of_New_Effective_Regulators_of_Oilseed_Rape_Growth_Among_Derivatives_of_Oxazole_and_Oxazolopyrimidine
79. Lutynska G.O., Biliavska L.O., Babych O.A., Tsygankova V.A., Babych A.G. The Monograph “Plant protection and bioregulation in modern agriculture” / Ed. “Diamond trading tour” Warszawa. Poland, 2019.- 100 p. ISBN: 978-83-66030-73-2.
80. The Monograph “Advances and Trends in Biotechnology and Genetics Vol. 3” / Eds. Dr. Tsygankova Victoria Anatolyivna; Prof. Dr. Lanzhuang Che. Book Publisher International. SCIENCEDOMAIN international Ltd. 2019. 166 p. DOI 10.9734/bpi/atbg/v3. ISBN9789389562460. (Indexed in Scopus).
81. Kolodiazhnyi O.I., Kolodiazhna A.O.. Stereoselective syntheses of sanshool derivatives. Phosphorus, Sulfur and Silicon and the Related Elements.- 2019.- Vol. 193. Р. 275-276. https://doi.org/10.1080/10426507.2018.1514404

82. Kolodiazhnyi O.I.. Stereochemistry of electrophilic and nucleophilic substitution at phosphorus. Phosphorus, Sulfur and Silicon and the Related Elements.- 2019. Vol.193. Р. 396-400. https://doi.org/10.1080/10426507.2018.1521409

83. Trush M.M., Kovalishyn V., Ocheretniuk A.D., Kobzar O.L., Kachaeva M.V., Brovarets V.S., Metelytsia L.O.. QSAR study of some 1,3-oxazolylphosphonium derivatives as new potent anti-Candida agents and their toxicity evaluation. Curr. Drug Discov. Technol.- 2019. – Vol.16(2). Р. 204-209. https://doi.org/10.2174/1570163815666180418145422
1. Rogalsky S., Bardeau J.-F., Makhno S., Babkina N., Tarasyuk O., Cherniavska T., Orlovska I., Kozyrovska N., Brovko O.. New proton conducting membrane based on bacterial cellulose/polyaniline nanocomposite film impregnated with guanidinium-based ionic liquid. Polymer. – 2018. – Т.142. – P.183-195. https://doi.org/10.1016/j.polymer.2018.03.032

2. Fatyeyeva K., Rogalsky S., Tarasyuk O., Chappey C., Marais S.. Vapour sorption and permeation behaviour of supported ionic liquid membranes: application for organic solvent/water separation. Reactive and functional polymers. – 2018. – Vol.130. – P.16-28. https://doi.org/10.1016/j.reactfunctpolym.2018.05.007
3. Vashchuk A., Rios de Anda A., Starostenko O., Grigoryeva O., Sotta P., Rogalsky S., Smertenko P., Fainleib A., Grande D.. Structure – property relationships in nanocomposites based on cyanate ester resins and 1-heptyl pyridinium tetrafluoroborate ionic liquid. Polymer. – 2018, Vol.148. – P.14-26. https://doi.org/10.1016/j.polymer.2018.06.015

4. Hodyna D., Kovalishyn V., Semenyuta I., Blagodatnyi V., Rogalsky S., Metelytsia L.. Imidazolium ionic liquids as effective antiseptics and disinfectants against drug resistant S. aureus: in silico and in vitro studies. Computational Biology and Chemistry. – 2018. – Vol.73. – P.127-138. https://doi.org/10.1016/j.compbiolchem.2018.01.012
5. Trush M.M., Kovalishyn V.V., Ocheretniuk A.D., Kalashnikova L.E., Prokopenko V.M., Holovchenko O.V., Kobzar O.L., Brovarets V.S., Metelytsia L.O.. New 1,3-oxazolylphosphonium Salts as Potential Biocides: QSAR Study, Synthesis, Antibacterial Activity and Toxicity Evaluation.. Letters in Drug Design & Discovery. – 2018.- Vol. 15 Issue:12. – P. 1259-1267. http://dx.doi.org/10.2174/1570180815666180219164334

6. I. Pokotylo, V. Kravets, J. Martinec, E. Ruelland. The phosphatidic acid paradox: Too many actions for one molecule class? Lessons from plants. Progress in Lipid Research.- 2018.- V.71.- P. 43-53. https://doi.org/10.1016/j.plipres.2018.05.003
7. Kovalishyn V., Grouleff J., Semenyuta I., Sinenko V.O., Slivchuk S.R., Hodyna D., Brovarets V., Blagodatny V., Poda G., Tetko I.V., Metelytsia L.. Rational design of isonicotinic acid hydrazide derivatives with anti-tubercular activity: Machine learning, molecular docking, synthesis and biological testing. Chem. Biol. & Drug Des.- 2018.- V.92 (№1).- P. 1272-1278. https://doi.org/10.1111/cbdd.13188
8. Kachaeva M.V., Hodyna D.M., Semenyuta I.V., Pilyo S.G., Prokopenko V.M., KovalishynV.V., Metelytsia L.O., Brovarets V.S.. Design, synthesis and evaluation of novel sulfonamides as potential anticancer agents. Comput. Biol. Chem.- 2018.- V.10 (№74).- P. 294-303. https://doi.org/10.1016/j.compbiolchem.2018.04.006

9. L.K. Patrylak, M.M. Krylova, O.P. Pertko, Yu.G. Voloshyna. Linear Hexane Isomerization over Ni-Containing Pentasils. J. Porous Mater.- 2018. Р. 861–868. https://doi.org/10.1007/s10934-018-0685-1
10. Iu. Little, E. Alorkpa, V. Khan, V. Povazhnyi, A.Vasiliev. Efficient porous adsorbent for removal of cesium from contaminated water. J. Porous Mater.- 2018.- Vol. 244.- Р. 55-63. https://doi.org/10.1007/s10934-018-0608-1
11. P.Le. Maout, J.-L. Wojkiewicz, N. Redon, C. Lahuec, F. Seguin, L. Dupont, S. Mikhaylov, Yu. Noskov, N. Ogurtsov, A. Pud. Polyaniline nanocomposites based sensor array for breath ammonia analysis. Portable e-nose approach to non-invasive diagnosis of chronic kidney disease. Sensors and Actuators B.- 2018.- Vol. 274.- Р. 616-626. https://doi.org/10.1016/j.snb.2018.07.178
12. Z. Hamouda, J.L. Wojkiewicz, A.A. Pud, L. Kone, S. Bergheul, T. Lasri. Magnetodielectric Nanocomposite Polymer-Based Dual-Band Flexible Antenna for Wearable Applications. IEEE Transactions on Antennas and Propagation.- 2018.- Vol. 66(7).- Р. 3271-3277. https://doi.org/10.1109/TAP.2018.2826573
13. Ogurtsov N.A., Bliznyuk V.N., Mamykin A.V., Kukla O.L., Piryatinski Yu.P., Pud A.A.. Improved Structural and Electronic Properties of Poly(3-Methylthiophene) in Nanocomposites with Poly(Vinylidene Fluoride). Effect of an Electroactive Template”. Physical Chemistry Chemical Physics.- 2018.- Vol. 20.- Р. 6450-6461. https://doi.org/10.1039/C7CP07604E
14. Trepyadko D., Korzh R, Kremenetskii V, Nasieka Iu., Naseka V., Stanovyi O., Bortyshevskii V.. Graphitic carbon sub-nitride: synthesis, structure, electron and proton transport, phо-toluminescence and thermoelectric proper-ties. Materials Chemistry and Physics. – 2018. – Vol. 209. – P. 95-106. http://dx.doi.org/10.1016/j.matchemphys.2018.01.069

15. I.O. Yaremchuk, L.V. Muzychka, O.B. Smolii, O.V. Kucher, S.V. Shishkina. Synthesis of novel 1,2-dihydro-pyrrolo[1,2-a]pyrazin-1(2H)-one derivatives. Tetrahedron Lett.- 2018.- Vol. 59, №5.- P.442-444 19. https://doi.org/10.1016/j.tetlet.2017.12.065

16. A.M. Zinchenko, L.V. Muzychka, O.V. Kucher, I.V. Sadkova, P.K. Mykhailiuk, O.B. Smolii. One-Pot Synthesis of 6-Aminopyrido[2,3-d]pyrimidin-7-ones. Eur. J. Org. Chem.- 2018.- № 47. DOI: 10.1002/ejoc.201801204.

17. Tarasenko K.V., Romanenko V.D., Sorochinsky A.E.. Condensation of diethyl fluoromethylphosphonate with esters: An alternative synthetic route to diethyl ?-fluoro-?-ketophosphonates. J. Fluorine Chem.- 2018.- Vol. 211.- Р. 124-128. https://doi.org/10.1016/j.jfluchem.2018.04.014

18. Vdovenko S.I., Gerus I.I., Pagacz M.-Kostrzewa, Wierzejewska M., Zhuk Y.I., Kukhar V.P.. Special feature of kinetics of ZcE isomerization of ?-N-methylaminovinyl trifluoromethyl ketone in Ar matrix exposed to UV radiation and spontaneous E Z isomerization of methyl-N-methylaminovinyl trifluoromethyl ketone. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy.- 2018.- Vol. 199.- Р. 130–140. https://doi.org/10.1016/j.saa.2018.03.049

19. Khlebnicova T.S., Piven Yu.A., Isakova V.G., Baranovsky A.V., Lakhvich F.A., Sorochinsky A.E., Gerus I.I. Regioselective Synthesis of Polyfluoroalkyl Substituted 6,7-Dihydrobenzisoxasolones. Journal of Heterocyclic Chemistry.- 2018.- Vol. 55.- Р. 1791-1797. http://dx.doi.org/10.1002/jhet.3218
20. Romanenko V.D., Kukhar V.P.. Phosphonate analogues of nucleoside polyphosphates. Arkivoc.- 2018.- part i.- Р.1-49 https://doi.org/10.24820/ark.5550190.p010.183.
21. Kondratov I.S., Bugera M.Ya., Tolmachova N.A., Daniliuc C.G., Haufe G.. Straightforward synthesis of fluorinated amino acids by Michael addition of ethyl bromodifluoroacetate to ?, ?-unsaturated ?-amino acid derivatives. Journal of Fluorine Chemistry.- 2018.- Vol. 211.- Р. 100-108. https://doi.org/10.1016/j.jfluchem.2018.03.014

22. Borysko P., Moroz Y.S., Vasylchenko O.V., Hurmach V.V., Starodubtseva A., Stefanishena, N. Nesteruk K., Zozulya S., Kondratov I.S., Grygorenko O.O.. Straightforward hit identification approach in fragment-based discovery of bromodomain-containing protein 4 (BRD4) inhibitors. Bioorganic & medicinal chemistry.- 2018.- Vol. 26, № 12.- Р. 3399-3405. https://doi.org/10.1016/j.bmc.2018.05.010
23. Kondratov I.S., Tolmachova N.A, Haufe G.. Diels–Alder Reaction in the Synthesis of Fluorinated (Hetero) Aromatic Compounds. European Journal of Organic Chemistry.- 2018.- (27-28).- Р. 3618-3647. https://doi.org/10.1002/ejoc.201800327

24. Gerus I.I., Balabon O.A., Pazenok S.V., Lui N., Kondratov I.S., Tarasenko K.V., Shaitanova E.N., Ivasyshyn, V.E. Kukhar V.P. Synthesis and Properties of Polyfunctional Cyclic ?-Alkoxy-Unsaturated Ketones Based on 4-Methylene-1,3-dioxolanes. European Journal of Organic Chemistry.- 2018.- (27-28).- Р. 3853-3861. https://doi.org/10.1002/ejoc.201800786

25. Tolmachova N.A., Kondratov I.S., Dolovanyuk V.G., Pridma S.O., Chernykh A.V., Daniliuc C.G., Haufe G.. Synthesis of new fluorinated proline analogues from polyfluoroalkyl ?-ketoacetals and ethyl isocyanoacetate. Chemical Communications.- 2018.- Vol. 54 (69).- Р. 9683-9686. https://doi.org/10.1039/C8CC05912H
26. Zyabrev V.S., Babii S.B.. Reactions of 5-mesyl-2-phenyl-4-tosyloxazole with N-, C-, and S-nucleophiles. Chem. Heter. Comp.- 2018. – Vol. 54, № 1. – Р. 93-95. https://doi.org/10.1007/s10593-018-2237-7

27. Frasinyuk M.S., Zhang W., Wyrebek P., Yu T., Xu X., Sviripa V.M., Bondarenko S.P., Xie Y., Ngo H.X., Morris A.J., Mohler J.L., Fiandalo M.V., Watt D.S., Liu C.. Developing antineoplastic agents that target peroxisomal enzymes: cytisine-linked isoflavonoids as inhibitors of hydroxysteroid 17-beta-dehydrogenase-4(HSD17B4). Organic, Biomolecular Chemistry.- 2017. – Vol. 15.- P.7623-7629. https://doi.org/10.1039/C7OB01584D
28. Solomyannyi R., Slivchuk S., Smee D., Choi J., Rusanov E., Zhirnov V., Brovarets V.. In vitro activity of the novel pyrimidines and their condensed derivatives against poliovirus. Current Bioactive Comp. 2018. – Vol.14, № 14. – P. 1-9. https://doi.org/10.2174/1573407214666180720120509
29. Kachkovsky A., Obernikhina N., Prostota Y., Naumenko A., Melnyk D., Yashchuk V.. Estimation of the basicity of the donor strength of terminal groups in cationic polymethine dyes. J. Molec. Structure.- 2018. – Vol.1154. – P. 606-618. https://doi.org/10.1016/j.molstruc.2017.10.051

30. Sinenko V.O., Slivchuk S.R., Brovarets V.S.. Lithiation of 2-Bromo-4-(1,3-dioxolan-2- yl)-13-thiazole. Current Chem. Letters.- 2018. – Vol. 8. – P.1-8. http://dx.doi.org/10.5267/j.ccl.2018.1.002
31. Kachaeva M.V., Pilyo S.G., Demydchuk B.A., Prokopenko V.M., Zhirnov V.V., Brovarets V.S. 4-Cyano-1,3-oxazole-5-sulfonamides as Novel Promising Anticancer Lead Compounds. Intern. J. Curr. Res.- 2018. – Vol. 10, № 5. – P. 69410-69425. https://www.researchgate.net/publication/326008659_4-CYANO-13-OXAZOLE_ANTICANCER_LEAD_COMPOUNDS
32. Tsygankova V., Andrusevich Y., Shompel O., Kopich V., Solomyanny R., Bondarenko O., Brovarets V.. Phytohormone-like effect of pyrimidine derivatives on regulation of vegetative growth of tomato. International J. of Botany Studies.- 2018. – Vol. 3, № 2. – P. 91-102.
33. Tsygankova V., Andrusevich Y., Kopich V., Shtompel O., Veligina Ye., Pilyo S., Kachaeva M., Kornienko A., Brovarets V.. Application of Oxazole and Oxazolopyrimidine as New Effective Regulatores of Oilseed Rape Growth. Sch. Bull.- 2018. – Vol. 4, № 3. – P. 301-312. http://dx.doi.org/10.21276/sb.2018.4.3.8
34. Chalyk B.A., Hrebeniuk K.V., Gavrilenko K.S., Shablykin O.V., Yanshyna O.O., Bash D., Mykhailik P.K., Liashuk O.S., Grygorenko O.O.. Synthesis of Bi- and Polyfunctional Isoxazoles from Amino Acid-Derived Halogenoximes and Active Methylene Nitriles. Eur. J. Org. Chem.- 2018. -Vol. 22. – P. 2753-2761. https://doi.org/10.1002/ejoc.201800311
35. Popova A.V., Bondarenko C.P., Frasinyuk M.S., Wen Zhang, Xie Yanji, Martin Zachary M., Cai Xianfeng, Fiandalo Michael V., Mohler James L., Liu Chunming, Watt David S., Sviripa V.M.. Efficient synthesis of aurone Mannich bases and evaluation of their antineoplastic activity in PC-3 prostate cancer cells. Chemical Papers, 2018. – Vol. 72, № 10. – P. 2443-2456. https://doi.org/10.1007/s11696-018-0485-8
36. Kachaeva M., Pilyo S., Popilnichenko S., Kornienko A., Rusanov E., Prokopenko V., Zyabrev V., Brovarets V.. Synthesis of fused heterocycles from 2-aryl-5-(chlorosulfonyl)oxazole-4-carboxylates and α-aminoakoles involving the Smiles rearrangement. Current Chem. Letters.- 2018. – Vol. 7, № 4. – P. 101-110. http://dx.doi.org/10.5267/j.ccl.2018.9.001
37. Brovarets V.S., Golovchenko O.V., Rusanov E.B., Rusanova J.A.. Crystal structure of diethyl {2,2,2-tri¬chloro-1-[2-(1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl)-4-methyl¬pentanamido]¬eth-yl}phospho¬nate. Acta Cryst.- 2018. – Vol. E74. – P. 915-917. https://doi.org/10.1107/S2056989018008277.
38. Tsygankova V.A., Andrusevich Ya.V., Shtompel O.I., Pilyo S.G., Kornienko A.M., Brovarets V.S.. Using of [1,3]oxazolo[5,4-d]pyrimidine and N-sulfonyl substituted of 1,3-oxazole to improve the growth of soybean seedlings. Chem. Res. J.- 2018. – Vol. 3 (2).- P. 165-173. https://www.researchgate.net/publication/325248643_Using_of_13oxazolo54-dpyrimidine_and_N-sulfonyl_substituted_of_13-oxazole_to_improve_the_growth_of_soybean_seedlings
39. Velihina Ye., Kachaeva M.V., Pilyo S.G., Zhirnov V.V., Brovarets V.S.. Synthesis, Characterization, and Vitro Anticancer Evaluation of 7-Piperazin-substituted [1,3]oxazolo[4,5-d]pyrimidines. Der Pharma Chemica.- 2018. – Vol. 10, № 9. – Р. 1-10. https://www.derpharmachemica.com/pharma-chemica/synthesis-characterization-and-in-vitro-anticancer-evaluation-of-7piperazinsubstituted-13oxazolo45dpyrimidines-15338.html
40. Tsygankova V.A., Andrusevich Ya.V., Shtompel O.I., Pilyo S.G., Kornienko A.M., Brovarets V.S.. Acceleration on vegetative growth of wheat (Triticum aestivum L.) using [1,3] oxazolo[5,4-d]pyrimidine and N-sulfonyl substituted 1,3-oxazole. Pharm. Chem. J., 2018. – Vol.5, № 2. – P. 167-175. https://tpcj.org/download/vol-5-iss-2-2018/TPCJ2018-05-02-167-175.pdf
41. Bogolyubsky A., Savych O., Zhemera A., Khomenko D., Brovarets V., Moroz Yu., Vybornyi M.. A facile one-pot parallel synthesis of 3-amino-1,2,4-triazoles. ACS Combinatorial Science, 2018. – Vol. 20, № 7.– P. 461-466. https://doi.org/10.1021/acscombsci.8b00060

42. Kachaeva M.V., Pilyo S.G., Zhirnov V.V., Brovarets V.S. Synthesis, characterization, and in vitro anticancer evaluation of 2- substituted 5-arylsulfonyl-1,3-oxazole-4-carbonitriles. Med. Chem. Res.- 2018. – Vol. 22, № 12 https://doi.org/10.1007/s00044-018-2265-y.
43. Mrug G.P., Demydchuk B.A., Bondarenko S.P., Sviripa V.M., Wyrebek P., Mohler J. L., Fiandalo M.V., Liu C., Frasinyuk M.S., Watt D.S.. A Direct Synthesis of 2-(?-Carboxyalkyl)-isoflavones from ortho-Hydroxylated Deoxybenzoins. Eur. J. Org. Chem.- 2018. – Vol. 39. – P. 5460- 5463. https://doi.org/10.1002/ejoc.201801171

44. Bondarenko S.P., Frasinyuk M.S., Mrug G.P., Vinogradova V.I., Khilya V.P.. Synthesis of Isoflavone-Anabasine Conjugates. Chem. Nat. Comp.- 2018.- Vol. 54, № 6. – P. 1068-1071. https://dx.doi.org/10.1007/s10600-018-2557-y.
45. Tsygankova V.A., Andrusevich Ya.V., Shtompel O.I., Solomyanny R.M., Hurenko A.O., Frasinyuk M.S., Mrug G.P., Shablykin O.V., Pilyo S.G., Kornienko A.M., Brovarets V.S.. Study of auxin-like and cytokinin-like activities of derivatives of pyrimidine, pyrazole, isoflavones, pyridine, oxazolopyrimidine and oxazole on haricot bean and pumpkin plants. Intern. J. Chem. Tech. Res.- 2018. -Vol. 11, № 10. – P. 174-190. http://dx.doi.org/10.20902/IJCTR.2018.111022
46. Velihina Ye.S., Kachaeva M.V., Pilyo S.G., Mitiukhin O.P., Zhirnov V.V., Brovarets V.S.. Synthesys, characterization and in vitro anticancer evaluation of 7-(1,4-diazepan)substituted [1,3]oxazolo[4,5]pyrimidines. Chem. Res. J.- 2018 Doi:10.1007/s00044-018-2265-y. https://www.researchgate.net/publication/333732106_Synthesis_Characterization_and_In_Vitro_Anticancer_Evaluation_of_7-14-Diazepan-_substituted_13oxazolo45-dpyrimidines
47. Shokol T.V., Gorbulenko N.V., Frasinyuk M.S., Khilya V.P.. Furo[2,3-h]chromones and pyrano[2’,3’:5,6]chromeno[4,3-b]pyridines based on natural isoflavones. Chem. Nat. Comp.- 2018. – Vol. 54, № 6. – Р. 1065-1067 https://dx.doi.org/10.1007/s10600-018-2556-z.
48. Popova A.V., Bondarenko S.P., Frasinyuk M.S.. Synthesis and aminomethylation of regioisomeric 6-hydroxy-4-methyl- and 4-hydroxy-6-methylaurones. Chem. Heter. Comp.- 2018. – Vol. 54, № 9. – P. 832- 839. https://doi.org/10.1007/s10593-018-2360-5
49. Bondarenko S.P., Frasinyuk M.S.. Observations from aminomethylation of 7-substituted 6-hydroxyaurones. Chem. Heter. Comp.- 2018. – Vol. 54, № 8. – P. 765-762. https://doi.org/10.1007/s10593-018-2347-2
50. Shokol T.V., Abdullaev E.N., Gorbulenko N.V., Frasinyuk M.S., Khilya V.P.. Synthesis and modification of 6-thiazolyl-4-methylumbelliferone. Chem. Nat. Comp. 2018. – Vol. 54, №3. – P. 439-442. https://doi.org/10.1007/s10600-018-2374-3
51. Tolmachova K., Moroz Y., Konovets A., Platonov M., Vasylchenko O., Borysko P., Zozulya S., Gryniukova A., Bogolubsky A., Pipko S., Mykhailiuk P., Brovarets V., Grygorenko O. . (Chlorosulfonyl)benzenesulfonyl Fluorides—Versatile Building Blocks for Combinatorial Chemistry: Design, Synthesis and Evaluation of a Covalent Inhibitor Library. ACS Comb. Sci.- 2018. – Vol. 20, № 11. – P. 672-680. https://doi.org/10.1021/acscombsci.8b00130

52. Adejuwon A.O., Tsygankova V.A., Alonge O.. Effect of cultivation conditions on activity of ?-amylase from a tropical strain Aspergillus Flavus Link. J. Microbiol. Biotechnol. Food Sci.- 2018. -Vol.7, № 6. – P. 571-575. https://www.researchgate.net/publication/325598705_Effect_of_cultivation_conditions_on_activity_of_a-amylase_from_a_tropical_strain_aspergillus_flavus_link
53. Tsygankova V.A., Andrusevich Ya.V., Shtompel O.I., Shablykin O.V., Hurenko A.O., Solomyanny R.M., Mrug G.P., Frasinyuk M.S., Pilyo S.G., Kornienko A.M., Brovarets V.S.. Auxin-like effect of derivatives of pyrimidine, pyrazole, isoflavones, pyridine, oxazolopyrimidine and oxazole on acceleration of vegetative growth of flax. Intern. J. Pharm. Tech. Res.- 2018. – Vol.11, № 3. – P. 274-286. https://www.researchgate.net/publication/326064558_Auxin-like_effect_of_derivatives_of_Pyrimidine_Pyrazole_Isoflavones_Pyridine_Oxazolopyrimidine_and_Oxazole_on_acceleration_of_Vegetative_growth_of_Flax
54. Zuzana Krckova, Daniela Kocourkova, Michal Danek, J itka Brouzdova, Premysl Pejchar, Martin Janda, Igor Pokotylo, Peter G. Ott, Olga Valentova, Jan Martinec . The Arabidopsis thaliana non-specific phospholipase C2 is involved in the response to Pseudomonas syringae attack. Annals of Botany.- 2018.- 121.- Р. 297–310. https://doi.org/10.1093/aob/mcx160
1. V.Kovalishyn,V.Brovarets,V.Blagodatnyi,I. Kopernyk,D. Hodyna,S.Chumachenko,O.Shablykin,O. Kozachenko,M. Vovk,M.Barus,M. Bratenko, L.Metelytsia. QSAR studies, synthesis and antibacterial assessment of new inhibitors against multidrug-resistant Mycobacterium tuberculosis. // Current Drug Discovery Technologies.- 2017.- V.14, N.1 .- P. 25-38. https://doi.org/10.2174/1570163813666161108125227
2. Maria M. Trush, Ivan V. Semenyuta, Sergey I. Vdovenko, Sergiy P. Rogalsky, Evgeniya O. Lobko, Larisa O. Metelytsia. Synthesis, spectroscopic and molecular docking studies of imidazolium and pyridinium based ionic liquids with HSA as potential antimicrobial agents. // Journal of Molecular Structure.-V.1137, 5.- 2017.- P. 692–699. https://doi.org/10.1016/j.molstruc.2017.02.079

3. Protasov Al., Bardeau JF., Morozovskaya I., Boretska M., Cherniavska T., Petrus L., Tarasyuk O., Metelytsia L., Kopernyk I., Kalashnikova L., Dzhuzha O., Rogalsky S. New promising antifouling agent based on polymeric biocide polyhexamethylene guanidine molybdate. // Environ. Toxicol Chem. 2017.- V. 36(9).- P. 2543-2551. https://doi.org/10.1002/etc.3782
4. Soares A., Estevao M.S., Marques M.M.B., Kovalishyn V., A.R.S. Latino D., Aires-de-Sousa J., Ramos J., Viveiros M., Martins F. Synthesis and Biological Evaluation of Hybrid 1,5- and 2,5-Disubstituted Indoles as Potentially New Antitubercular Agents. // Medicinal chemistry.- 2017.- 13 (5), Р. 439-447. https://doi.org/10.2174/1573406413666170209144003
5. Kovalishyn V., Abramenko N., Kopernyk I., Charochkina L., Metelytsia L., Tetko I.V., Peijnenburg W.,Kustov L. Modelling the toxicity of a large set of metal and metal oxide nanoparticles using the OCHEM platform. // Food and Chemical Toxicology.- 2017. – 17. – S.0278-6915. https://doi.org/10.1016/j.fct.2017.08.008
6. Kolodiazhnyi O.I.,Kolodiazhna A.O. Nucleophilic substitution at phosphorus: stereochemistry and Mechanisms. // Tetrahedron: Asymmetry. 2017.- 28,N11; Р. 1651-1674. https://doi.org/10.1016/j.tetasy.2017.10.022

7. Kolodiazhnyi O.I., Kolodiazhna A.O. Stereochemistry of nucleophilic substitution at trivalent phosphorus. // Phosphorus, Sulfur, and Silicon and the Related Elements.- 2017.- V. 192, N 6.- P. 621-633. https://doi.org/10.1080/10426507.2017.1284842

8. Ghamrawi S.,Bouchara J.-P.,Tarasyuk O.,Rogalsky S.,Lyoshina L.,Bulko O.,Bardeau J.-F. Promising Silicones Modified with Cationic Biocides for the Development of Antimicrobial Medical Devices // Materials Science and Engineering C. – 2017. – V. 75. – P. 969-979. https://doi.org/10.1016/j.msec.2017.03.013
9. McLaughlin K.,Folorunso A.,Deeni Y.,Foster D.,Hapca S.,Immoor C.,Koza A.,Mohammed I.,Moshynets J.,Rogalsky S.,Zawadzki K.,Spiers A. Biofilm formation and cellulose expression by Bordetella avium 197N, the causative agent of bordetellosis in birds and an opportunistic respiratory pathogen in humans. // Research in Microbiology. – 2017. – V. 168(5). – P. 419-430. https://doi.org/10.1016/j.resmic.2017.01.002
10. Fainleib A.Vashchuk A.Starostenko O.Grigoryeva O.Rogalsky S.Nguyen T.-T., Grande D. Nanoporous Polymer Films of Cyanate Ester Resins Designed by Using Ionic Liquids as Porogens // Nanoscale Research Letters.- 2017.- 12:126 doi: 10.1186/s11671-017-1900-8. Epub 2017 Feb 17. https://doi.org/10.1186/s11671-017-1900-8
11. Kenneth Seaton, Iuliia Little, Cameron Tate, Ray Mohseni, Marina Roginskaya, Volodymyr Povazhniy, Aleksey Vasiliev. Adsorption of cesium on silica gel containing embedded phosphotungstic acid // Microporous and Mesoporous Materials.- 2017.- V. 244.- Р. 55-66. https://doi.org/10.1016/j.micromeso.2017.02.025

12. Yu.G. Voloshyna,O.P. Pertko,S.V. Konovalov, L.K. Patrylak. Benzene as a by-product of toluene with methanol transformation on the basic zeolite catalysts and its presumable origin // Adsorption Science and Technology.- 2017. – V. 35.- issue 7-8.- P. 700-705. http://dx.doi.org/10.1177/0263617417705963
13. R. Korzh, V. Bortyshevskii. Primary Reactions of Lignite-Water Slurry Gasification under the Supercritical Fluids in the Electric Field // The Journal of Supercritical Fluids. – 2017. – V. 127. – P. 166-175. https://doi.org/10.1016/j.supflu.2017.04.004

14. Muzychka O.V., Kobzar O.L.,Popova A.V., Frasinyuk M.S.,Vovk A.I. Carboxylated aurone derivatives as potent inhibitors of xanthine oxidase // Bioorg. Med. Chem. 2017.– 25.– P. 3606–3613. https://doi.org/10.1016/j.bmc.2017.04.048

15. Khilya O.V., Milokhov D.S., Kononets L.A., Kobzar O.L.,Vovk A.I.,Volovenko Yu.M.. Synthesis and evaluation of new 2,6-diamino-5-hetarylpyrimidines as inhibitors of dihydrofolate reductase // Monatsh. Chem.-2017. https://doi.org/10.1007/s00706-017-2032-7
16. Bohdan A. Chalyk, Andrei A.Isakov, Maryna V. Butko, Kateryna V. Hrebeniuk, Olena V. Savych, Olexandr V. Kucher, Konstantin S. Gavrilenko, Tetiana V. Druzherko, Vladimir S. Yarmolchuk, Sergey Zozulya, Pavel K. Mykhailiuk Synthesis of 6-Azaspiro[4.3]alkanes Innovative Scaffolds for Drug Discovery // Eur. J. Org. Chem.- 2017.- № 31.- P.4530-4542. https://doi.org/10.1002/ejoc.201700536

17. Lyubov V. Muzychka, Iryna O. Yaremchuk, Oksana V. Muzychka, Oleg B. Smolii. 7-Substituted pyrrolo[2,3-d]-pyrimidines for the synthesis of new 1-deazapyrimido[1,2,3-cd]purines // French-Ukrainian J. Chem. 2017.- V.5, №2.- P.15-23. https://doi.org/10.17721/fujcV5I2P15-23
18. Anna N. Zinchenko, Lyubov V. Muzychka, Igor I. Biletskii, Oleg B. Smolii. Synthesis of new 4-amino-substituted 7-iminopyrido[2,3-d]pyrimidines // Chem. Heterocycl. Comp.- 2017.- V.53, №5.- P. 589-596. https://doi.org/10.1007/s10593-017-2096-7
19. Kondratov I.S.,Logvinenko I.G.,Tolmachova N.A.,Morev R.N.,Kliachyna M.A.,Clausen F.,Daniliuc C.,Haufe G. 2-Amino-4-(trifluoromethoxy)butanoic acid – first ?-aminoacid containing a CF3O-group in aliphatic position // Organic and Biomolecular Chemistry.- 2017.-15.- Р.672-679. http://dx.doi.org/10.1039/C6OB02436J
20. Feskov I.O.,Chernykh A.V.,Kondratov I.S.,Klyachina M.,Daniliuc C.G.,Haufe G. Cyclobutyl-Containing Rigid Analogues of Threonine: Synthesis and Physical Chemical Properties // Journal of Organic Chemistry.- 2017.- 82 (23), Р. 12863–12868. https://doi.org/10.1016/j.jfluchem.2022.109990

21. Romanenko V.D.,Kukhar V.P. Phosphonate analogues of nucleoside polyphosphates // Arkivoc.-2017.- (i).- Р. 1-49. http://dx.doi.org/10.24820/ark.5550190.p010.183
22. Vdovenko S.I.,Zhuk Yu.I.,Kukhar V.P.,Pagacz-Kostrzewab M.,WierzejewskabM.,Daniliuc C.-G. The conformational analysis of push–pull enaminones using Fourier transform IR and NMR spectroscopy, and quantum chemical calculations. VI. ?-N-Methylaminovinyl trifluoromethyl ketone ?-methyl-?-N-methylaminovinyl trifluoromethyl ketone // Journal of Molecular Structure.- 2017.-1128.- №. 1-3, Р.741–753. https://doi.org/10.1016/j.molstruc.2016.09.049

23. Borisova T.,PozdnyakovaN.,Shaitanova E.,Dudarenko M.,Haufe G.,Kukhar V. Effects of new fluorinated analogues of GABA, pregabalin bioisosters, on the ambient level and exocytotic release of [3H]GABA from rat brain nerve terminals. // Bioorganic & Medicinal Chemistry.- 2017.-25.- Р.759–764. https://doi.org/10.1016/j.bmc.2016.11.052

24. Demydchuk B.A., Rusanov E.B., Rusanova S.A., Brovarets V.S. Regioselective synthesis of bicyclic by 1,3,5-triazepine system starting from tetrachloro-2-aza-1,3-butadienes // Current Chemistry Letters.- 2017. – 6. – P. 49-54. http://dx.doi.org/10.5267/j.ccl.2017.2.001
25. Kachaeva M V., Kornienko Andriї N., Prokopenko Volodymyr M., Zhirnov Victor V., Prichard Mark N., Keith Kathy A., Yang Guang, Wang Hsu-Kun, Benerjee N.Sanjil, Chow Louise T., Broker Thomas R., Brovarets Volodymyr S. In Vitro Activity of Novel 1,3-Oxazole Derivatives against Human Papilloma-virus // Ibnosina J. of Med. And Biomed. Sciences.- 2017. – V.9, №4. – P.111-118. https://www.thieme-connect.com/products/ejournals/pdf/10.4103/ijmbs.ijmbs_9_17.pdf
26. Tsygankova V., Andrusevich Y., Shtompel O., Myroljubov O., Hurenko A., Solomyuny R., Mrug G., Frasinyuk M., Shablykin O., Brovarets V. Study of auxin, cytokinin and gibberellin – like activity of heterocyclic compounds derivatives of pyrimidin, pyridine, pyrazole and isoflavones // Eur. J. Biotechn. and Biosience.- 2016. – V.4, №12. – P. 29-44. https://www.researchgate.net/publication/311934046_Study_of_auxin_cytokinin_and_gibberellin-like_activity_of_heterocyclic_compounds_derivatives_of_pyrimidine_pyridine_pyrazole_and_isoflavones
27. Holovchenko O., Naumenko A., Vydzhak R., Abdurakhmanova E., Prostota Ya., Kachkovsky O., Brovarets V. Electronik and spectrel properties of phosphonium ylides-betaines, derivatives of 2-oxazoline-5-one // Eur. Chem. Bull., 2017. – V.6, № 9. – Р. 380-392. https://mail.bibliomed.org/?mno=43762
28. Popova A.V., Bondarenko S.P., Podobii E.V., Frasinyuk M.S., Vinogradova V.I. Synthesis of flavonoid derivatives of cytisine. 5. Aminomethylation of 6-hydroxyaurones // Chemistry of Natural Compounds.- 2017. – Vol. 53, No.4. – P. 603-607. https://doi.org/10.1007/s10600-017-2096-y
29. Sendiuk V.A., Pavlenko E.L., Dmytrenko O.P.,
Kulish M.P., Viniychuk O.O., Prostota Ya.O., Kachkovsky O.D. Interaction of solitons on 2-dimensional branched ?-electron surface of graphene ribbons // International Journal of Quantum Chemistry.- 2017, electronic “early bird publication”. https://doi.org/10.1002/qua.25454
30. Conceicao D.S., Ferreira D.P., Prostota Y., Kachkovsky O.D., Ferreira L.F. Vieira, Santos P.F. Longwavelength absorbing fluorescent polymethine dyes derived from the 6-(N,N-diethylamino)-1,2,3,4-tetrahydroxanthylium system // Journal of photochemistry and Photobiology A: Chemistry.- 2017. – V. 347. – P. 218-226. https://doi.org/10.1016/j.jphotochem.2017.07.045

31. Kobzar P.Y., Pavlenko E.L., Brusentsov V.A., Dmytrenko O.P., Kulish N.P., Bricks J.L., Slominskii Yu.L., Kurdyukov V.V., Tolmachev O.I., Kachkovsky O.D. Comparative Study of Electronic Structure Cyanine Bases versus Parent Cationic Cyanines // J. Advanc. Phys.- 2017. –V.6. – P. 1-12. http://dx.doi.org/10.1166/jap.2017.1347
32. Pavlenko O.L., Sendiuk V.A., Dmytrenko O.P., Kulish M.P., Sheludko E.V., Kachkovsky O.D., Ilchenko O.O., Shlapatska V.V. Electron and vibration structure of fluorine – containing polyamides film under high energetic electron irradiation // BAHT (Problems of Atomic Science and Technology).- 2017. – №4 (110). – P. 17-20. https://www.researchgate.net/publication/319434720_Electron_and_vibration_structure_of_fluorine-containing_polyamide_film_under_high_energetic_electron_irradiation
33. Shaydyuk Y.O., Levchenko S.M., Kurhuzenkau S.A., Anderson D., Masunov A.E., Kachkovsky O.D., Slominsky Y.L., Bricks J.L., Belfield K.D., Bondar M.V. Linear photo-physics, two-photon absorption and femtosecond transient absorption spectroscopy of styryl dye // Journal of Luminescence.- 2017. – V.183. – P. 360-367. https://doi.org/10.1016/j.jlumin.2016.11.073
34. Kachkovsky A., Obernikhina N., Prostota Ya., Naumenko A., Melnyk D., Yashchuk V. Estimation of the basicity of the donor strength of terminal groups in cationic polymethine dyes // Journal of Molecular Structure.- 2018. – 15. – P. 606-618. https://doi.org/10.1016/j.molstruc.2017.10.051

35. Shablykin O.V., Merzhyievsky D.O., Shablykina O.V. The interaction of homophtalic anhydride with (triphenylphosphoranylidene)acetates // French-Ukr. J. Chem. – 2017. – Vol.5, №2. – P. 62-67. https://doi.org/10.17721/fujcV5I2P62-67
36. M. Derevyanchuk, S.Kretynin, O. Iakovenko, R. Litvinovskaуа, Y. Blume, J. Martinec, V. Khripach, V. Kravets. Effect of 24-epibrassinolide on Brassica napus alternative respiratory pathway, guard cells movements and phospholipid signaling under salt stress // Steroids. – 2017. V. 117. – P. 16–24. https://doi.org/10.1016/j.steroids.2016.11.006
37. Krchkova Z., Kotsurkova D., Danek M., Browzdova J., Peykhar P., Yanda M., Pokotilo I., Ot P., Valentyova O., Martynets Ya. The Arabidopsis thaliana non-specifc phospholipase C2 is involved in the response to Pseudomonas syringae attack // Annals of Botany. https://doi.org/10.1093/aob/mcx160
38. S.K. Kohli, N. Hunda, R. Sharna, H. Kaur, A.K. Thurkul, S. Avrora, V. Кravets, R. Bhardvaj Multigene engineering strategies for crop improvement // Chapter 4 in book: Mechanisms behind phytohormonal signalling and crop abiotic stress response. Р. 61-88.
39. Pud A.A., Fedorenko E.A., Vretik L.O., Ogurtsov N.A., Nikolayeva O.A., Kruglyak O.S., Noskov Yu.V. New nanocomposites of polystyrene with polyaniline doped with lauryl sulfuric acid // Nanoscale Research Letters.- 2017.- № 12, paper 493 (11 pages). https://doi.org/10.1186/s11671-017-2265-8
40. Dimitriev O.P., Grynko D.O., Fedoryak A.M., Doroshenko T.P., Kratzer M., Teichert C., Noskov Yu.V.,Ogurtsov N.A., Pud A.A., Balaz P., Balaz M., Tesinsky M. Macroscopic versus microscopic photovoltaic response of heterojunctions based on mechanochemically prepared nanopowders of kesterite and n-type semiconductors // Semiconductor Physics, Quantum Electronics & Optoelectronics.- 2017.- V. 20, N 4.- P. 418-423. https://www.researchgate.net/publication/322715210_Macroscopic_versus_microscopic_photovoltaic_response_of_heterojunctions_based_on_mechanochemically_prepared_nanopowders_of_kesterite_and_n-type_semiconductors
41. Hamouda Z., Wojkiewicz J-L., Pud A.A., Kone L., Bergheul S., Lasri T. A Flexible UWB Organic Antenna for Wearable Technologies Application // IET Microwaves Antennas & Propagation, accepted manuscript.- 2017. https://doi.org/10.1049/iet-map.2017.0189
42. Hamouda Z., Wojkiewicz J-L., Pud A.A., Kone L., Bergheul S., Lasri T. Design, fabrication and characterization of a new wideband antenna based on a Polyaniline/Carbon coated Cobalt composite // Proceedings of the 11th European Conference on Antennas and Propagation (EUCAP), 19-24 March 2017, Р. 2130-2133.
43. Wojkiewicz J.-L, Redon N., Pud A., Mikhaylov S., Ogurtsov N., Noskov Y., Collard C., Li W. Hybrid and Bio Nanocomposites for Ultrasensitive Ammonia Sensors // Proceedings.- 2017.- V.1, №4.- paper 407 (5 pages). https://doi.org/10.3390/proceedings1040407
44. Mikhaylov S., Pud A., Wojkiewicz J.-L., Coddeville P. UV-light Induced Solid-phase Photodegradation in PANI Nanocomposites // Proceedings of Nanomaterials: Application & Properties.- 2017.- V. 6, 03NNSA09. http://dx.doi.org/10.1109/NAP.2017.8190257
45. Myronyuk I., Pud A., Mamykin A., Kukla A. The Specificity of the Core-shell Polyvinylidene/Polyaniline Nanocomposite Sensing Applications // Proceedings of Nanomaterials : Application & Properties.- 2017.- V. 6, 03NNSA19. http://dx.doi.org/10.1007/s13204-021-01812-9
46. L. Zheleznyi,G. Pop,O. Papeykin,I. Venger, L. Bodachivska. Development of compositions of urea greases on aminoamides of fatty acids // Eastern-European Journal of Enterprise Technologies. – 2017, №3/6(87).– С. 9-15. https://doi.org/10.15587/1729-4061.2017.99580
47. Iukhymenko V.V., Chernyak V.Ya.,Hamazin D.K.,Levko D.S.,Bortyshevskyy V.A.,Korzh R.V. Rotating Gliding Discharge Submerged in Liquid // Problems of Atomic Science and Technology. – 2017.-Р. 136-139. http://dspace.nbuv.gov.ua/handle/123456789/122151
48. Hamazin D.K.,Iukhymenko V.V., Chernyak V.Ya.,Prysiazhna O.V.,Martysh E.V.,Bortyshevskyy V.A., Korzh R.V. Properties of Secondary Discharge in Plasma-Liquid System Based on Rotating Gliding Discharge // Problems of Atomic Science and Technology. – 2017.-Р. 167-170. https://www.researchgate.net/publication/315720644_Properties_of_secondary_discharge_in_plasma-liquid_system_based_on_rotating_gliding_discharge
49. V. Tsygankova, Y. Andrusevich, O. Shtompel, O. Romaniuk, M. Yaikova, A. Hurenko, R. Solomyanny, E. Abdurakhmanova, S. Klyuchko, O. Holovchenko, O. Bondarenko, V. Brovarets. Application of Synthetic Low Molecular Weight Heterocyclic Compounds Derivatives of Pyrimidine, Pyrazole and Oxazole in Agricultural Biotechnology as a New Plant Growth Regulating Substances. // Int. J. Med. Biotechnol. & Genetics. – 2017. – S2: 002.– P. 10-32. http://dx.doi.org/10.19070/2379-1020-SI02002
50. Victoria Tsygankova, Elena Shysha, Anatoly Galkin, Lyudmila Biliavska, Galina Iutynska, Alla Yemets, Yaroslav Blume. Impact of Microbial Biostimulants on Induction of Callusogenesis and Organogenesis in the Isolated Tissue Culture of Wheat in vitro. // J. Med. Plants. Stud. – 2017. – V. 5, Issue 3. – P. 155-164. https://www.researchgate.net/publication/317041975_Impact_of_microbial_biostimulants_on_induction_of_callusogenesis_and_organogenesis_in_the_isolated_tissue_culture_of_wheat_in_vitro
51. Ocheretniuk A.D., Kobzar O.L., Mischenko I.M., Vovk A.I N-Phenacylthiazolium Salts as Inhibitors of Cholinesterases // French-Ukrainian Journal of Chemistry, 2017, Vol 5, №2.- P. 1-14.
52. Buldenko V.M., Kobzar O.L.,Trush V.V.,Drapailo A.B., Kalchenko V.I.,Vovk A.I. Sulfonyl-bridged Calix[4]arene as an Inhibitor of Protein Tyrosine Phosphatases // French-Ukrainian Journal of Chemistry, 2017, Vol. 5, №2.- P. 144-151. https://doi.org/10.17721/fujcV5I2P144-151
1. Hodyna D. Antibacterial activity of imidazolium-based ionic liquids investigated by QSAR modeling and experimental studies / D. Hodyna, V. Kovalishyn, S. Rogalsky[et al.] // Chem. Biol. Drug Des. — 2016. — 88, №3. — P. 422–433. https://doi.org/10.1111/cbdd.12770
2. Tanchuk V. Y. A new, improved hybrid scoring function for molecular docking and scoring based on autodock and autodock vina / V. Y. Tanchuk, V. O. Tanin, A. I. Vovk, G. Poda // Chem. Biol. Drug. Des. — 2016. — 87, №4. — P. 618–625. https://doi.org/10.1111/cbdd.12697
3. Hodyna D. Efficient antimicrobial activity and reduced toxicity of 1-dodecyl-3-methylimidazolium tetrafluoroborate ionic liquid/beta-cyclodextrin complex / D. Hodyna, J.-F. Bardeau, L. Metelytsia[et al.] // Chemical Engineering Journal. — 2016. — 284. — P. 1136–1145. https://doi.org/10.1016/j.cej.2015.09.041
4. A domino reaction for the synthesis of 2H-pyrano-[4″,3″,2″:4′,5′]chromeno[2′,3′:4,5]thieno-[2,3-b]pyridin-2-ones / S. P. Bondarenko, I. V. Zhitnetskyi, S. V. Semenov, M. S. Frasinyuk // Chem. Heterocycl Comp. — 2016. — 52, №4. — P. 262–266. https://doi.org/10.1007/s10593-016-1872-0
5. Mrug G. P. Inverse electron demand diels–alder reactions with aminomethyl derivatives of 3-arylhydroxycoumarins / G. P. Mrug, K. M. Kondratyuk, S. P. Bondarenko, M. S. Frasinyuk // Chem. Heterocycl Comp. — 2016. — 52, №7. — P. 460–466. https://doi.org/10.1007/s10593-016-1907-6
6. Popova A. V. Synthesis and properties of 2-benzylidene-8,9-dihydro-7H-furo[2,3-f][1,3]benzoxazin-3(2h)-one derivatives / A. V. Popova, S. P. Bondarenko, M. S. Frasinyuk // Chem Heterocycl Comp. — 2016. — 52, №8. — P. 592–600. https://doi.org/10.1007/s10593-016-1937-0
7. Shablykin O. V. Interaction of 2-aryl-4-(dichloromethylidene)-1,3-oxazol-5(4H)-ones with methyl 2-isocyanoacetate / O. V. Shablykin, V. M. Prokopenko, V. S. Brovarets // Chem. Heterocycl Comp. — 2016. — 52, №6. — P. 424–426. https://doi.org/10.1007/s10593-016-1905-8
8. Bondarenko S. P. New aloperine–isoflavone conjugates / S. P. Bondarenko, M. S. Frasinyuk, V. P. Khilya // Chem. Nat. Compd. — 2016. — 52, №4. — P. 615–619. https://doi.org/10.1007/s10600-016-1723-3
9. Bondarenko S. P. Synthesis of 4-aryl-5-[2-hydroxy-4-(2-cytisin-12-ylethoxy)phenyl]isoxazoles / S. P. Bondarenko, M. S. Frasinyuk, V. I. Vinogradova, V. P. Khilya // Chem. Nat. Compd. — 2016. — 52, №3. — P. 463–467. https://doi.org/10.1007/s10600-016-1673-9
10. Bondarenko S. P. Reductive amination as an aminomethylation method for isoflavone ring b / S. P. Bondarenko, I. V. Zhitnetskii, S. V. Semenov, M. S. Frasinyuk // Chem. Nat. Compd. — 2016. — 52, №5. — P. 802–806. https://doi.org/10.1007/s10600-016-1782-5
11. Popova A. V. New heterocyclic pyrano[2′,3′:5,6]chromeno[3,2-c]pyridin-4-ones and furo[2′,3′:5,6]chromeno[3,2-c]pyridin-3(2H)-ones synthesized via a hetero-diels–alder reaction / A. V. Popova, G. P. Mrug, K. M. Kondratyuk[et al.] // Chem. Nat. Compd. — 2016. — 52, №6. — P. 1000–1004. http://dx.doi.org/10.1007/s10600-016-1846-6
12. Frasinyuk M. S. Antineoplastic isoflavonoids derived from intermediate ortho-quinone methides generated from mannich bases / M. S. Frasinyuk, G. P. Mrug, S. P. Bondarenko[et al.] // Chem. Med. Chem. — 2016. — 11, №6. — P. 600–611. https://doi.org/10.1002/cmdc.201600008
13. Semenyuta I. 1,3-oxazole derivatives as potential anticancer agents: computer modeling and experimental study / I. Semenyuta, V. Kovalishyn, V. Tanchuk[et al.] // Comput. Biol. Chem. — 2016. — 65. — P. 8–15. https://doi.org/10.1016/j.compbiolchem.2016.09.012

14. Кazymyr І. Patrylak. Coke alternate movement in faujasite based catalysts deactivated from butene alkylation / Кazymyr І. Patrylak, Lyubov К. Patrylak, Olexandra P. Pertko[et al.] // Current Catalysis. — 2016. — 5, №2. — P. 108–115. https://doi.org/10.2174/2211544705666160322235846
15. Hodyna D. Imidazolium ionic liquids as potential anti-candida inhibitors: QSAR modeling and experimental studies / D. Hodyna, V. Kovalishyn, S. Rogalsky[et al.] // Curr. Drug. Discov. Technol. — 2016. — 13, №2. — P. 109–119. https://doi.org/10.2174/1570163813666160510122201
16. Kalachova T. The inhibition of basal phosphoinositide-dependent phospholipase c activity in arabidopsis suspension cells by abscisic or salicylic acid acts as a signalling hub accounting for an important overlap in transcriptome remodelling induced by these hormones / T. Kalachova, R. Puga-Freitas, V. Kravets[et al.] // Environmental and Experimental Botany. — 2016. — 123. — P. 37–49. https://doi.org/10.1016/j.envexpbot.2015.11.003
17. Haidai O. Improvement of performance characteristics of ethanol motor fuels through use of additives based on nanoscale carbon clusters / O. Haidai, V. Pilyavskiy, Y. Shelud’ko, Y. Polunkin // Current Catalysis. — 2016. — 0, №6. — P. 3–10. http://dx.doi.org/10.21303/2461-4262.2016.00213
18. Khimach N. Recycling of carbone oxides (CO, CO2) conversion into methanol at atmospheric pressure over mechanochemical achtivated CuOo-ZnO-Al2O3 catalyst / N. Khimach, V. Yevdokymenko, I. Polunkin // Eureka: Physics and Engineering. — 2016. — 0, №6. — P. 11–18. https://doi.org/10.21303/2461-4262.2016.00210
19. Tsygankova V. Using of new microbial bio stimulants for obtaining in vitro new lines of Triticum aestivum L. cells resistant to nematode H. avenae. / V. Tsygankova, E. Shysha, Y. Andrusevich[et al.] // Eur. J. Biotechn. and Biosc. — 2016. — 4, №4. — P. 41–53. https://www.academia.edu/24969084/Using_of_new_microbial_biostimulants_for_obtaining_in_vitro_new_lines_of_Triticum_aestivum_L_cells_resistant_to_nematode_H_avenae
20. Chernykh A. V. Synthesis and physical-chemical properties of cis- and trans-1-amino-3-fluoro-3-methylcyclobutanecarboxylic acids / A. V. Chernykh, I. O. Feskov, A. V. Chernykh[et al.] // Eur. J. Org. Chem. — 2016. — 2016, №28. — P. 4782–4786. https://doi.org/10.1002/ejoc.201600953
21. Tsygankova V. Study of growth regulating activity derivatives of [1,3]Oxazolo[5,4-d]pyrimidine and N-Sulfonyl substituted of 1,3-Oxazole on soybean, wheat, flax and pumpkin plants. / V. Tsygankova, Y. Andrusevich, O. Shtompel[et al.] // Int. J. Chem. Stud. — 2016. — 4, №5. — P. 106–120. https://www.researchgate.net/publication/309741763_Study_of_growth_regulating_activity_derivatives_of_13Oxazolo54-dpyrimidine_and_N-Sulfonyl_substituted_of_13-Oxazole_on_soybean_wheat_flax_and_pumpkin_plants
22. L.O.Biliavska. Application of new microbial plant resistance/plant growth protection inducers for increasing chinese cabbage plant tolerance against parasitic nematode heterodera schachtii schmidt / L.O.Biliavska, V.A.Tsygankova, V.E.Kozyritska[et al.] // Int. J. Res. Biosciences. — 2016. — V.5.- N2. – P. 64-82. https://www.ijrbs.in/index.php/ijrbs/article/view/203
23. Tsygankova V. Screening of five and six-membered nitrogen-containing heterocyclic compounds as new effective stimulants of linum usitatissimum l. organogenesis in vitro / V. Tsygankova, Bayer O. O, Andrusevich Ya. V. [et al.] // Intern. J. Med. Biotechnology Genetics. — 2016. — P. 1–9. http://dx.doi.org/10.19070/2379-1020-SI02001
24. Melnyk A. K. An EPR spin probe study of liposomes from sunflower and soybean phospholipids / A. K. Melnyk, O. V. Sukhoveev, L. A. Kononets[et al.] // J. Liposome Res — 2016. — 26, №1. — P. 80–86. https://doi.org/10.3109/08982104.2015.1039031
25. Rogalsky S. Structural, thermal and antibacterial properties of polyamide 11/polymeric biocide polyhexamethylene guanidine dodecylbenzenesulfonate composites / S. Rogalsky, J.-F. Bardeau, H. Wu[et al.] // J Mater Sci. — 2016. — 51, №16. — P. 7716–7730. https://doi.org/10.1007/s10853-016-0054-x
26. Adetola O. Modification of silica gel by heteropolyacids / O. Adetola, L. Golovko, A. Vasiliev // Key Engineering Materials — 2016. — 689. — P. 126–132. https://doi.org/10.4028/www.scientific.net/KEM.689.126
27. Sokolyuk P. A. Synthesis of diverse pyrazole-4-sulfonyl chlorides starting from 2-(benzylthio)malonaldehyde / P. A. Sokolyuk, I. S. Kondratov, O. V. Gavrylenko, A. A. Tolmachov // Mol. Divers. — 2016. — 20, №1. — P. 1–7. https://doi.org/10.1007/s11030-015-9633-z
28. Kolodiazhna A. O. Synthesis, properties and stereochemistry of 2-halo-1,2-lambda-5-oxaphosphetanes / A. O. Kolodiazhna, O. I. Kolodiazhnyi // Molecules — 2016. — 21, №10. — P. 1371–1392. https://doi.org/10.3390/molecules21101371
29. Grynko D. A. Hybrid solar cell on a carbon fiber / D. A. Grynko, A. N. Fedoryak, P. S. Smertenko[et al.] // Nanoscale Res. Lett. — 2016. — 11, №1. — P. 265. https://doi.org/10.1186/s11671-016-1469-7
30. Korzh R. Supercritical water as nanomedium for gasification of lignite-water suspension / R. Korzh, V. Bortyshevskyi // NanoScale Res. Lett. — 2016. — 11, №1. — P. 255–263. https://doi.org/10.1186/s11671-016-1458-x
31. Belik M. Y. Resolution of fluorinated aminophosphonic acid enantiomers by precolumn derivatization with following reverse phase liquid chromatography / M. Y. Belik, O. Yegorov, A. E. Sorochinsky, V. P. Kukhar // Phosphorus, Sulfur, and Silicon — 2016. — 191, №3. — P. 423–429. https://doi.org/10.1080/10426507.2015.1094656

32. Kolodiazhnyi O. I. Multiple stereoselectivity in organophosphorus chemistry / O. I. Kolodiazhnyi, A. O. Kolodiazhna // Phosphorus, Sulfur, and Silicon. — 2016. — 191, №3. — P. 444–458. https://doi.org/10.1080/10426507.2015.1091831

33. Povolotskii M. I. Molecular and electronic structure of 1,3,2-diazaphosphinine derivatives / M. I. Povolotskii, O. V. Shablykin, E. B. Rusanov, A. B. Rozhenko // Phosporus, Sulfur, and Silicon. — 2016. — 191, №3. — P. 399–404. https://doi.org/10.1080/10426507.2015.1091827

34. Derevyanchuk M. Brassinosteroid-induced de novo protein synthesis in zea mays under salinity and bioinformatic approach for identification of heat shock proteins / M. Derevyanchuk, R. Litvinovskaya, V. Khripach, V. Kravets // Plant Growth Regul. — 2016. — 78, №3. — P. 297–305. https://doi.org/10.1007/s10725-015-0093-3
35. Kolesnikov Y. S. Molecular mechanisms of gravity perception and signal transduction in plants / Y. S. Kolesnikov, S. V. Kretynin, I. D. Volotovsky[et al.] // Protoplasma. — 2016. — 253, №4. — P. 987–1004. https://doi.org/10.1007/s00709-015-0859-5
36. Chen G. Development of nanostructure–activity relationships assisting the nanomaterial hazard categorization for risk assessment and regulatory decision-making / G. Chen, W. J. G. M. Peijnenburg, V. Kovalishyn, M. G. Vijver // RSC Adv. — 2016. — 6, №57. — P. 52227–52235. https://doi.org/10.1039/C6RA06159A
37. Noskov Y. Acid-dopant effects in the formation and properties of polycarbonate-polyaniline composites / Y. Noskov, S. Mikhaylov, P. Coddeville[et al.] // Synthetic Metals. — 2016. — 217. — P. 266–275. https://doi.org/10.1016/j.synthmet.2016.04.015
38. Kucher O. V. Lipase kinetic enantiomeric resolution of 1-heteroarylethanols / O. V. Kucher, A. O. Kolodyazhnaya, O. B. Smolii[et al.] // Tetrahedron: Asymmetry. — 2016. — 27, №7–8. — P. 341–345. https://doi.org/10.1016/j.tetasy.2016.02.012

39. Ogurtsov N. A. Influence of dispersed nanoparticles on the kinetics of formation and molecular mass of polyaniline / N. A. Ogurtsov, S. D. Mikhaylov, P. Coddeville[et al.] // J. Phys. Chem. B. — 2016. — 120, №38. — P. 10106–10113. https://doi.org/10.1021/acs.jpcb.6b05944

40. Ogurtsov N. A. Evolution and interdependence of structure and properties of nanocomposites of multiwall carbon nanotubes with polyaniline / N. A. Ogurtsov, Y. V. Noskov, V. N. Bliznyuk[et al.] // J. Phys. Chem. C. — 2016. — 120, №1. — P. 230–242. https://doi.org/10.1021/acs.jpcc.5b08524

41. Korzh R. Primary reactions of lignite-water slurry gasification under the supercritical conditions / R. Korzh, V. Bortyshevskyi // J. Supercritical Fluids. — 2016. — 117. — P. 64–71. https://doi.org/10.1016/j.supflu.2016.05.013

42. Kolodiazhnyi O. I. Wiley: asymmetric synthesis in organophosphorus chemistry: synthetic methods, catalysis and applications / O. I. Kolodiazhnyi. — 2016. First published:30 September 2016 Print ISBN:9783527341504 |Online ISBN:9783527341542 |DOI:10.1002/9783527341542. https://onlinelibrary.wiley.com/doi/book/10.1002/9783527341542

43. Mikhaylov S. The PANI-DBSA content and dispersing solvent as influencing parameters in sensing performances of TiO 2/PANI-DBSA hybrid nanocomposites to ammonia / S. Mikhaylov, N. A. Ogurtsov, N. Redon[et al.] // RSC Adv. — 2016. — 6, №86. — P. 82625–82634. https://doi.org/10.1039/C6RA12693F
44. Tsygankova V. Study of auxin, cytokinin and gibberellin-like activity of heterocyclic compounds derivatives of pyrimidine, pyridine, pyrazole and isoflavones / V. Tsygankova, Y. Andrusevich, O. Shtompel[et al.] // 2016. — 4, №12. — P. 29–44. https://www.researchgate.net/publication/311934046_Study_of_auxin_cytokinin_and_gibberellin-like_activity_of_heterocyclic_compounds_derivatives_of_pyrimidine_pyridine_pyrazole_and_isoflavones
45. Tsygankova V. A. Stimulating effect of five and six-membered heterocyclic compounds on seed germination and vegetative growth of maize (Zea mays L.) / V. A. Tsygankova, Y. Andrusevich, O. Shtompel[et al.] // 2016. — 1, №4. — P. 1–14. https://www.researchgate.net/publication/309012218_Stimulating_effect_of_five_and_six-membered_heterocyclic_compounds_on_seed_germination_and_vegetative_growth_of_maize_Zea_mays_L
1. Borisova T. Synthesis of new fluorinated analogs of gaba, pregabalin bioisosteres, and their effects on [3H]gaba uptake by rat brain nerve terminals / T. Borisova, N. Pozdnyakova, E. Shaitanova[et al.] // Bioorg. Med. Chem. — 2015. — 23, №15. — P. 4316–4323. https://doi.org/10.1016/j.bmc.2015.06.038

2. Chernykh A. V. Synthesis and physicochemical properties of 3-fluorocyclobutylamines / A. V. Chernykh, D. S. Radchenko, A. V. Chernykh[et al.] // Eur. J. Org. Chem. — 2015. — 2015, №29. — P. 6466–6471. https://doi.org/10.1002/ejoc.201500746

3. Chumachenko S. A. Interaction of 5-(morpholin-4-yl)-2-(4-phthal-imidobutyl)- and 5-(morpholin-4-yl)-2-(5-phthal-imidopentyl)-1,3-oxazole-4-carbonitriles with hydrazine hydrate / S. A. Chumachenko, O. V. Shablykin, V. S. Brovarets // Chem Heterocycl Comp. — 2015. — 50, №12. — P. 1727–1730. https://doi.org/10.1007/s10593-015-1644-2
4. Dahi A. Water sorption properties of room-temperature ionic liquids over the whole range of water activity and molecular states of water in these media / A. Dahi, K. Fatyeyeva, C. Chappey[et al.] // RSC Adv. — 2015. — 5, №94. — P. 76927–76938. https://doi.org/10.1039/C5RA14066H
5. Derevyanchuk M. Effect of 24-epibrassinolide on arabidopsis thaliana alternative respiratory pathway under salt stress / M. Derevyanchuk, R. Litvinovskaya, V. Khripach[et al.] // Acta Physiol Plant. — 2015. — 37, №10. — P. 215. https://doi.org/10.1007/s11738-015-1967-8
6. Frasinyuk M. S. Synthesis and tautomerization of hydroxylated isoflavones bearing heterocyclic hemi-aminals / M. S. Frasinyuk, S. P. Bondarenko, V. P. Khilya[et al.] // Org. Biomol. Chem. — 2015. — 13, №4. — P. 1053–1067. https://doi.org/10.1007/s11738-015-1967-8
7. Frasinyuk M. S. Development of 6H-chromeno[3,4-c]pyrido[3′,2′:4,5]thieno[2,3-e]pyridazin-6-ones as par-4 secretagogues / M. S. Frasinyuk, S. P. Bondarenko, V. M. Sviripa[et al.] // Tetrahedron Lett. — 2015. — 56, №23. — P. 3382–3384. https://doi.org/10.1016/j.tetlet.2015.01.028

8. Frasinyuk M. S. Application of mannich bases to the synthesis of hydroxymethylated isoflavonoids as potential antineoplastic agents / M. S. Frasinyuk, G. P. Mrug, S. P. Bondarenko[et al.] // Org. Biomol. Chem. — 2015. — 13, №46. — P. 11292–11301. https://doi.org/10.1039/C5OB01828E
9. Grynko D. O. Multifunctional role of nanostructured cds interfacial layers in hybrid solar cells / D. O. Grynko, O. M. Fedoryak, P. S. Smertenko[et al.] // J Nanosci Nanotechnol. — 2015. — 15, №1. — P. 752–758. https://doi.org/10.1166/jnn.2015.9171
10. Hamouda Z. Dual-band elliptical planar conductive polymer antenna printed on a flexible substrate / Z. Hamouda, J. L. Wojkiewicz, A. A. Pud[et al.] // IEEE Transactions on Antennas and Propagation — 2015. — 63, №12. — P. 5864–5867. https://doi.org/10.1109/TAP.2015.2479643
11. Kalachova T. Importance of phosphoinositide-dependent signaling pathways in the control of gene expression in resting cells and in response to phytohormones / T. Kalachova, V. Kravets, A. Zachowski, E. Ruelland // Plant Signal Behav. — 2015. — 10, №5. — P. e1019983. https://doi.org/10.1080/15592324.2015.1019983
12. Kobzar O. L. Phosphonate derivatives of tetraazamacrocycles as new inhibitors of protein tyrosine phosphatases / O. L. Kobzar, M. V. Shevchuk, A. N. Lyashenko[et al.] // Org. Biomol. Chem. — 2015. — 13, №27. — P. 7437–7444. https://doi.org/10.1039/C5OB00713E
13. Kobzar O. L. Polycarboxylic fullerene derivatives as protein tyrosine phosphatase inhibitors / O. L. Kobzar, V. V. Trush, V. Y. Tanchuk[et al.] // Mendeleev Comm. — 2015. — 25, №3. — P. 199–201. https://doi.org/10.1016/j.mencom.2015.05.013
14. Kolodiazhna A. O. Synthesis and properties of four-membered phosphorus heterocycles-2-fluoro-1,2lambda5-oxaphosphetanes / A. O. Kolodiazhna, O. I. Kolodiazhnyi // Phosph., Sulfur, Silicon Relat. Elem. — 2015. — 190, №12. — P. 2232–2245. http://dx.doi.org/10.1080/10426507.2015.1054485

15. Kolodiazhna O. O. Synthesis of anti-cis-phosphiranes / O. O. Kolodiazhna, O. I. Kolodiazhnyi // Phosph., Sulfur, Silicon Relat. Elem. — 2015. — 190, №7. — P. 1192–1200. https://doi.org/10.1080/10426507.2014.979988

16. Kondratov I. S. Synthesis of trifluoromethyl-containing polysubstituted aromatic compounds by Diels–Alder reaction of ethyl 3-benzamido-2-oxo-6-(trifluoromethyl)-2H-pyran-5-carboxylate / I. S. Kondratov, N. A. Tolmachova, V. G. Dolovanyuk[et al.] // Eur. J. Org. Chem. — 2015. — 2015, №11. — P. 2482–2491. https://doi.org/10.1002/ejoc.201500032

17. Kovalishyn V. Efficient variable selection batch pruning algorithm for artificial neural networks / V. Kovalishyn, G. Poda // Chemometrics and Intelligent Lab. Sys. — 2015. — 149, Part B. — P. 10–16. https://doi.org/10.1016/j.chemolab.2015.10.005
18. Lukashuk O. I. Introduction of chiral 2-(aminoalkyl) substituents into 5-amino-1,3-oxazol-4-ylphosphonic acid derivatives and their use in phosphonodipeptide synthesis / O. I. Lukashuk, E. R. Abdurakhmanova, K. M. Kondratyuk[et al.] // RSC Adv. — 2015. — 5, №15. — P. 11198–11206. https://doi.org/10.1039/C4RA13819H
19. Mikhaylov S. Ammonia/amine electronic gas sensors based on hybrid polyaniline–tio2 nanocomposites. the effects of titania and the surface active doping acid / S. Mikhaylov, N. Ogurtsov, Y. Noskov[et al.] // RSC Adv. — 2015. — 5, №26. — P. 20218–20226. https://doi.org/10.1039/C4RA16121A
20. Mkrtchyan G. Molecular mechanisms of the non-coenzyme action of thiamin in brain: biochemical, structural and pathway analysis / G. Mkrtchyan, V. Aleshin, Y. Parkhomenko[et al.] // Sci Rep. — 2015. — 5. — P. 12583. https://doi.org/10.1038/srep12583
21. Ogurtsov N. A. Effect of multiwalled carbon nanotubes on the kinetics of the aniline polymerization: the semi-quantitative ocp approach / N. A. Ogurtsov, Y. V. Noskov, A. A. Pud // J. Phys. Chem. B. — 2015. — 119, №15. — P. 5055–5061. https://doi.org/10.1021/jp511665q

22. Pud A. “Anion-chromic” interactions of emeraldine base with hydroxide and halide anions in the solid polymer matrix / A. Pud, I. Duboriz, Y. Piryatinski, O. Dimitriev // Synthetic Metals. — 2015. — 209. — P. 232–239. https://doi.org/10.1016/j.synthmet.2015.07.034
23. Ruelland E. Role of phospholipid signalling in plant environmental responses / E. Ruelland, V. Kravets, M. Derevyanchuk[et al.] // Environmental and Experimental Botany. — 2015. — 114. — P. 129–143. https://doi.org/10.1016/j.envexpbot.2014.08.009
24. Tanchuk V. Y. A new scoring function for molecular docking based on autodock and autodock vina / V. Y. Tanchuk, V. O. Tanin, A. I. Vovk, G. Poda // Curr Drug Discov Technol. — 2015. — 12, №3. — P. 170–178. https://doi.org/10.2174/1570163812666150825110208
25. Tarasevych A. V. High temperature sublimation of alpha-amino acids: a realistic prebiotic process leading to large enantiomeric excess / A. V. Tarasevych, A. E. Sorochinsky, V. P. Kukhar, J.-C. Guillemin // Chem. Commun. — 2015. — 51, №32. — P. 7054–7057. https://doi.org/10.1039/C5CC00254K
26. Tarasevych A. V. Attrition-induced spontaneous chiral amplification of the gamma polymorphic modification of glycine / A. V. Tarasevych, A. E. Sorochinsky, V. P. Kukhar[et al.] // Cryst. Eng. Comm. — 2015. — 17, №7. — P. 1513–1517. https://doi.org/10.1039/C4CE02434F
27. Trush V. V. Phosphonate monoesters on a thiacalix[4]arene framework as potential inhibitors of protein tyrosine phosphatase 1B / V. V. Trush, S. G. Kharchenko, V. Y. Tanchuk[et al.] // Org. Biomol. Chem. — 2015. — 13, №33. — P. 8803–8806. https://doi.org/10.1039/C5OB01247C
28. Tsygankova V. Genetic control and phytohormonal regulation of plant embryogenesis / V. Tsygankova // Int. J. Medical Biotechnology Genetics (I)MBG — 2015. — P. 9–20. https://www.researchgate.net/publication/271802545_Genetic_Control_and_Phytohormonal_Regulation_of_Plant_Embryogenesis
29. Yagupolskii Y. L. Alternative synthetic routes to hydrofluoroolefins / Y. L. Yagupolskii, N. V. Pavlenko, S. V. Shelyazhenko[et al.] // Journal of Fluorine Chemistry. — 2015. — 179. — P. 134–141. https://doi.org/10.1016/j.jfluchem.2015.08.001

30. Zahanich I. Phenoxymethyl 1,3-oxazoles and 1,2,4-oxadiazoles as potent and selective agonists of free fatty acid receptor 1 (GPR40) / I. Zahanich, I. Kondratov, V. Naumchyk[et al.] // Bioorg. Med. Chem. Lett. — 2015. — 25, №16. — P. 3105–3111. https://doi.org/10.1016/j.bmcl.2015.06.018

31. Zhirnov V. V. The osmotic resistance, and zeta potential responses of human erythrocytes to transmembrane modification of Ca2+ fluxes in the presence of the imposed low rate radiation field of 90Sr / V. V. Zhirnov, I. N. Iakovenko // Int. J. Radiat. Biol. — 2015. — 91, №1. — P. 117–126. https://doi.org/10.3109/09553002.2014.950716
© 2026 IBOPC NAS of Ukraine