The Department of Chemistry of Bioactive Nitrogen Containing Heterocyclic Bases was established in 1960 at the Institute of Organic Chemistry of the Academy of Sciences of the Ukrainian SSR by merging part of the Department of Organophosphorus Compounds with the Department of Pyrimidine Bases. In 1987, the department was transferred to the newly established Institute of Bioorganic Chemistry of the Academy of Sciences of the Ukrainian SSR. From 1987 to 2009, the department was headed by Doctor of Chemical Sciences, Professor Borys Drach, under whose leadership the department became one of the largest in the Institute. Since 2009, the department has been headed by a Corresponding Member of the NAS of Ukraine, Doctor of Chemical Sciences, Professor, Laureate of the State Prize of Ukraine in Science and Technology, Volodymyr Brovarets, who expanded the scope of the department towards exploration of the relationship between heterocyclic compounds synthesised in the department and their bioactivity.
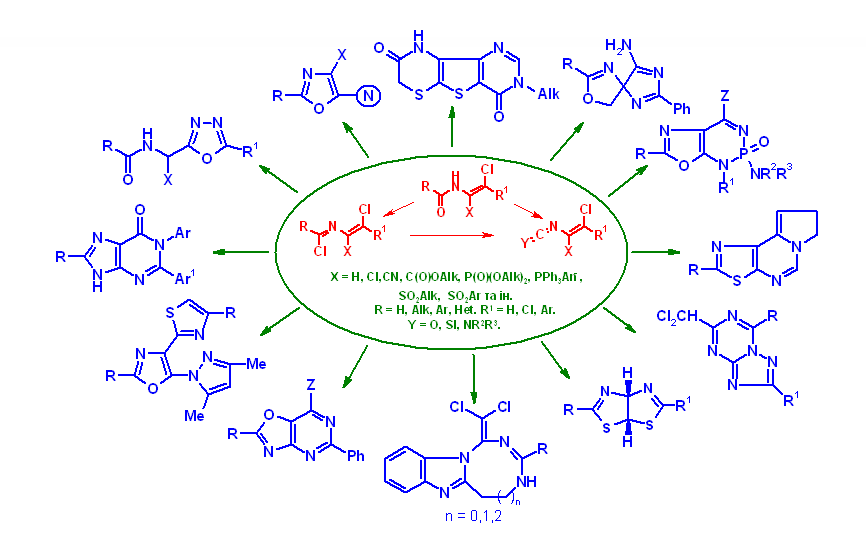
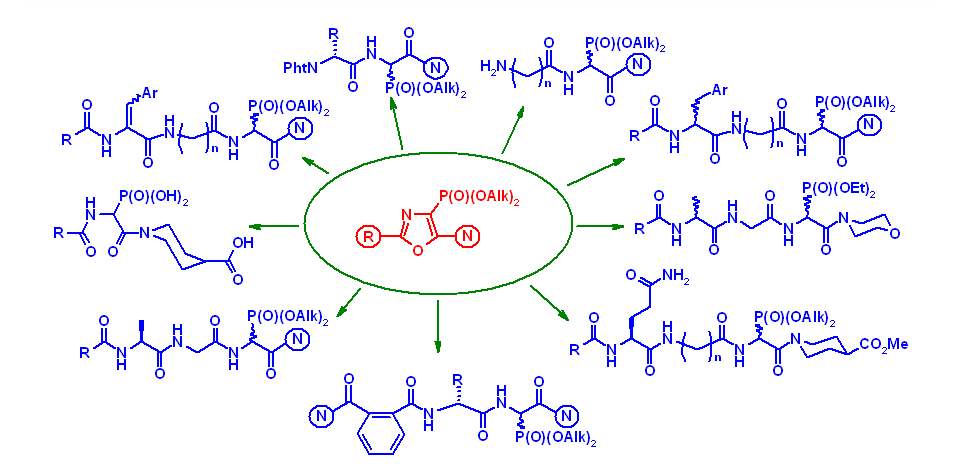
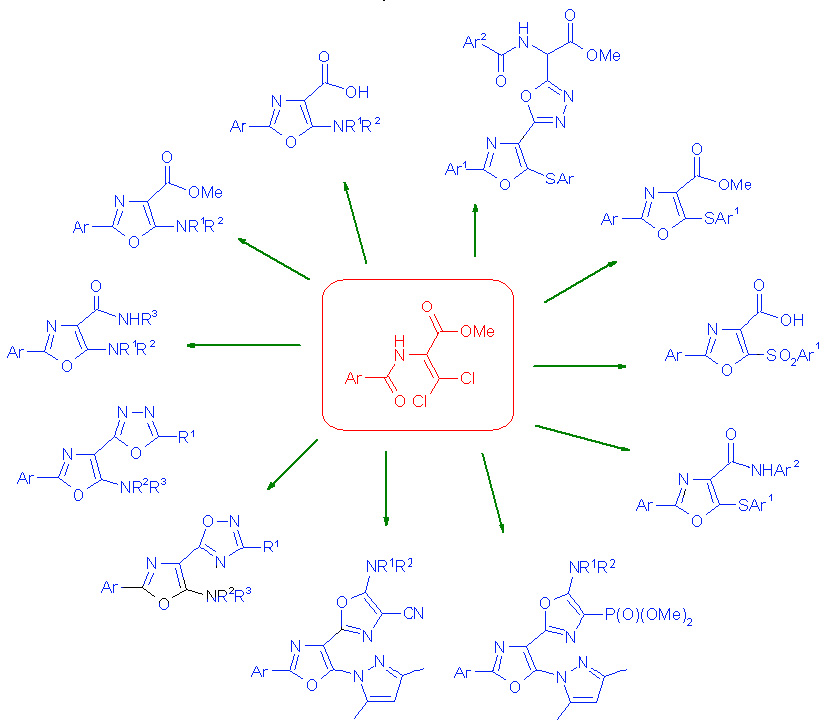
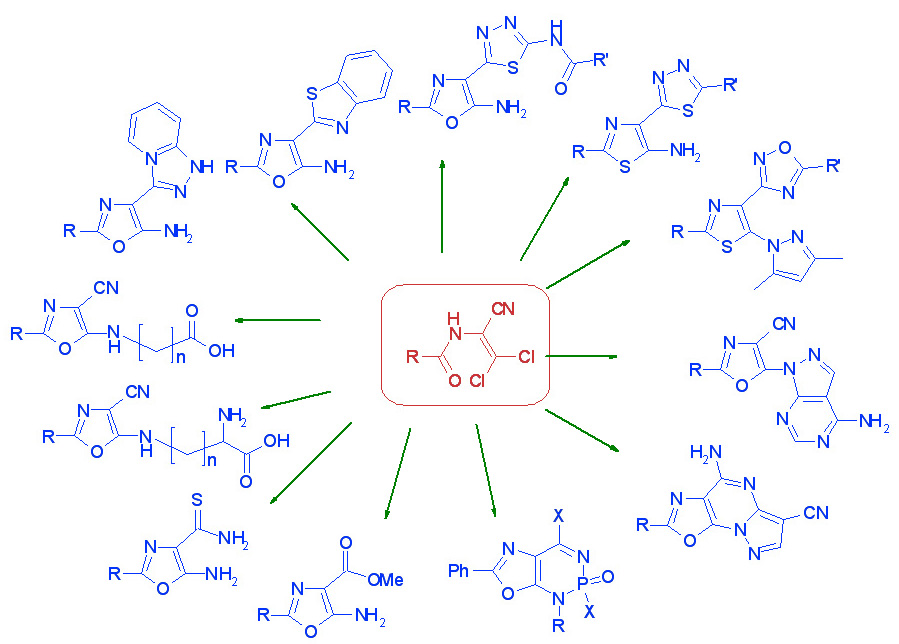
The main directions of the Department's scientific activity include the development of preparative methods for the synthesis of potential heterocyclic bioregulators, with the aim of discovering effective anticancer, antiviral drugs, enzyme inhibitors, pesticides, and plant growth regulators. To this end, the Department's scientists use original acyclic reagents that allow for the regioselective introduction of nitrogen-, sulfur- and phosphorus-containing pharmacophore groups into five- and six-membered nitrogenous heterocycles and their condensed derivatives. The Department also searches for and studies new approaches to the synthesis and modification of natural flavonoids and their analogues. The Department employs methods of fine organic synthesis, as well as spectral and X-ray diffraction studies and biological testing of synthesised compounds by specialists from our Institute and other research institutions. The Department conducts fundamental studies of the electronic and spectral properties of nitrogen heterocycles, in particular, their interaction with biologically active centres of biological targets, as well as quantum chemical modelling of biological processes at the molecular level. A promising area of the Department's activity is the search for new effective and environmentally friendly plant growth regulators based on synthetic five- and six-membered low-molecular-weight heterocyclic compounds with the prospect of their introduction into agricultural practice to improve the growth and development of commercially important plant crops, increase their yields, enhance the immune defence of plants against fungal, bacterial, viral and parasitic organisms while reducing the use of highly toxic chemicals, increasing plant resistance to abiotic stressors (drought, soil salinity, extreme temperatures, high intensity of solar radiation and nutrient deficiencies).
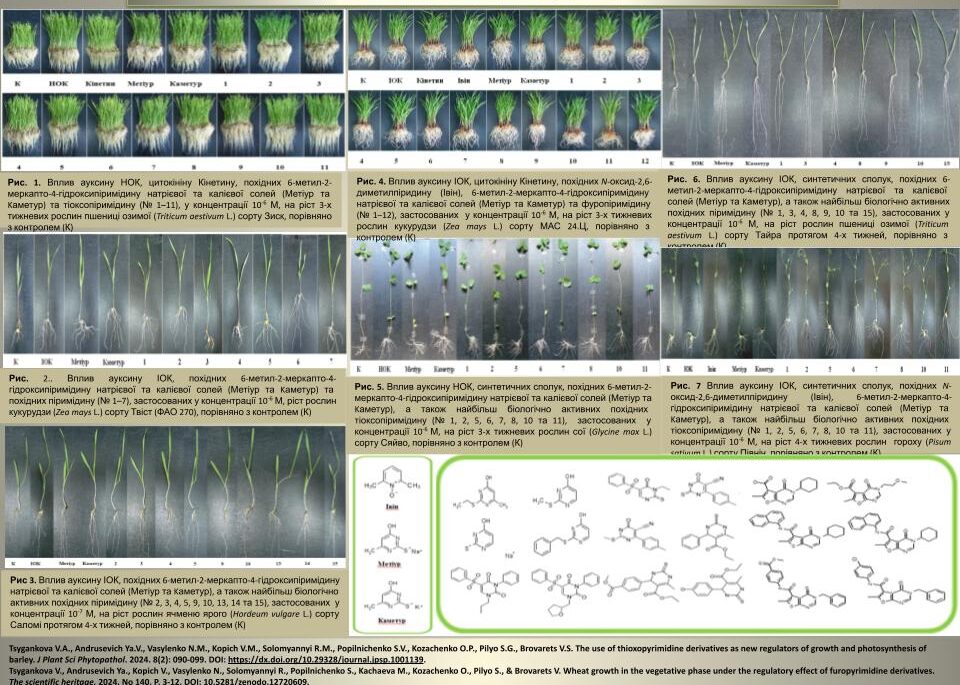
The most important scientific achievements of the Department in 2024
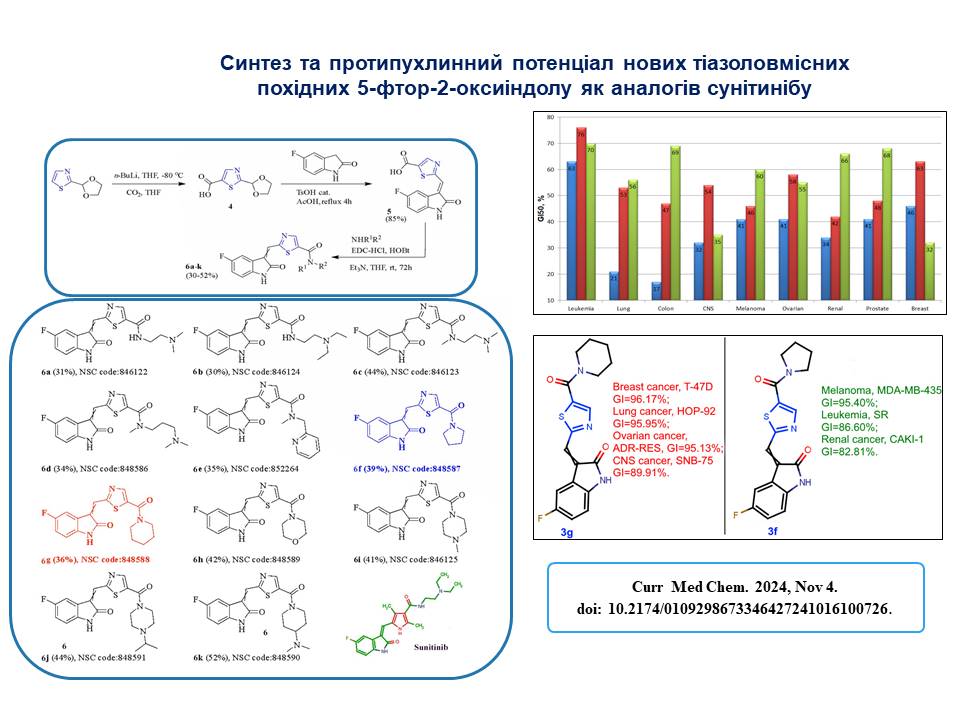
We have designed, synthesised and investigated 12 new thiazole-containing derivatives of 5-fluoro-1,3-dihydro-2H-indole-2-one as analogues of Sunitinib and studied their antitumour activity against NCI-60 cancer cell lines.
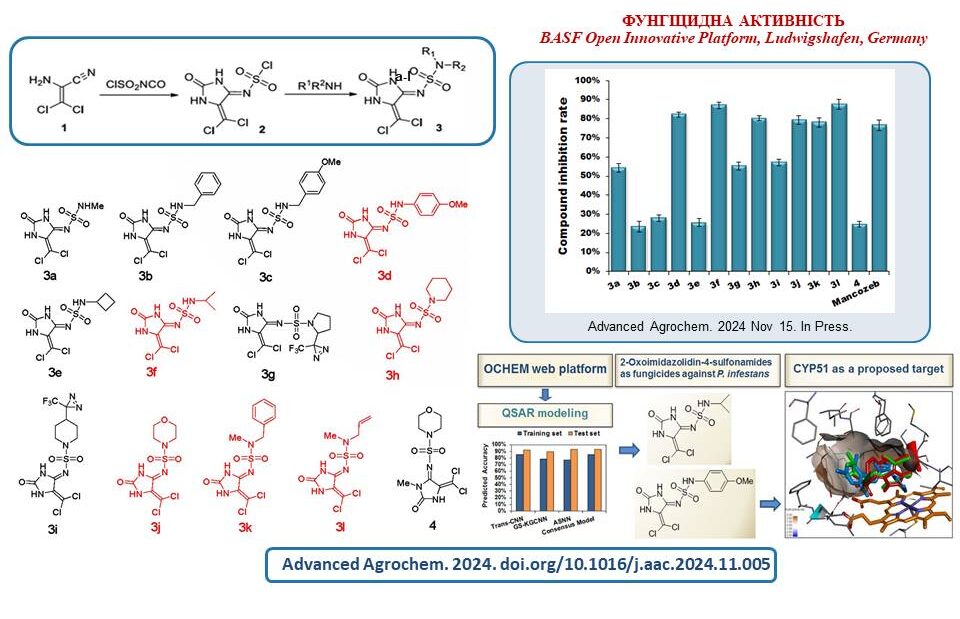
Based on the results of computer predictions and experimental data, several 2-oxoimidazolidine-4-sulfonamides were proposed as new fungicides for the control of Phytophthora infestans of agricultural plants. The results showed a low level of risk associated with using these compounds for the ecosystem and human health compared to the known classes of plant antiparasitic agents.
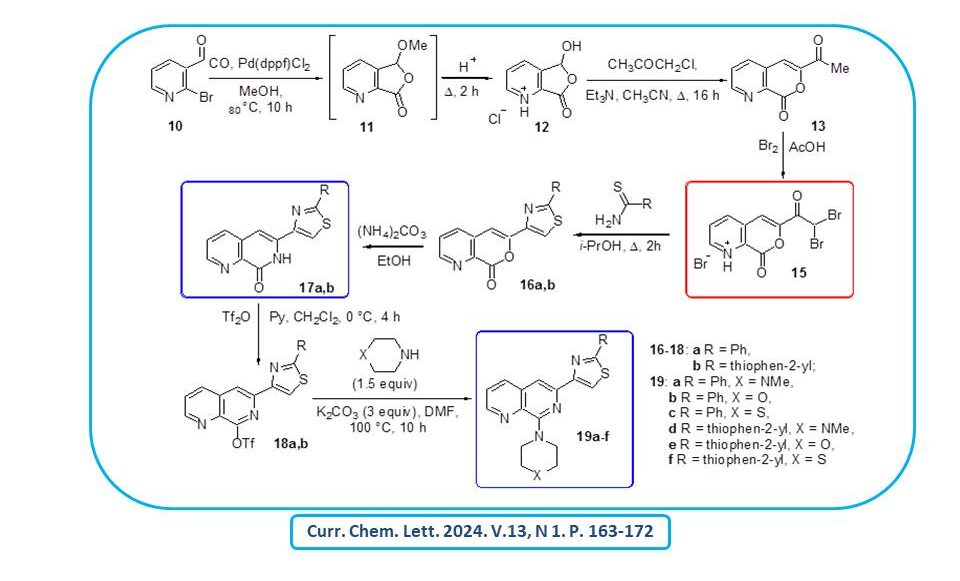
Manifold new 8-amino-6-(2-R-thiazol-4-yl)-1,7-naphthyridines, previously unavailable heterocyclic derivatives with promising biological activity, were obtained.
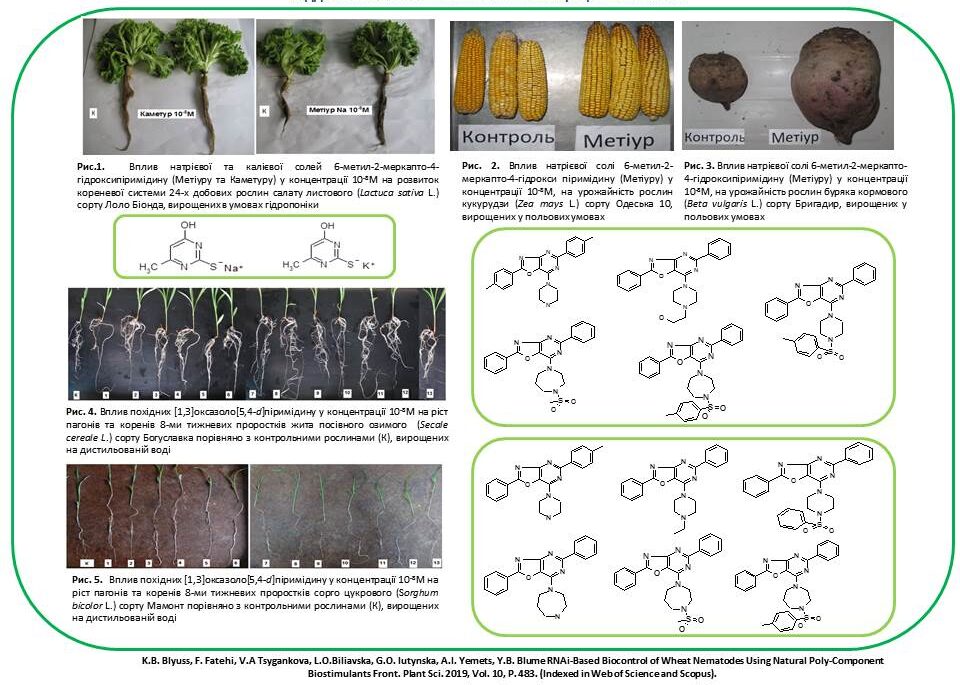
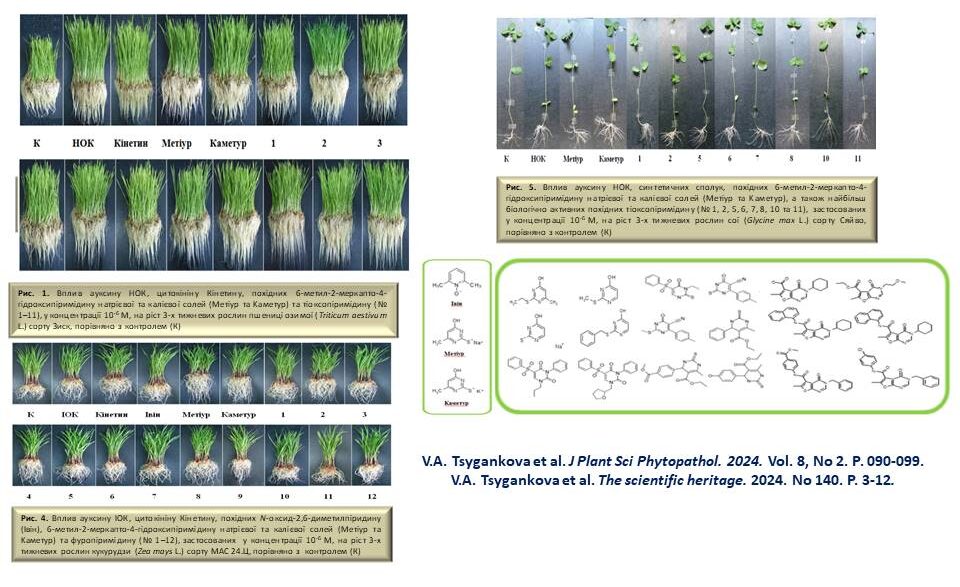
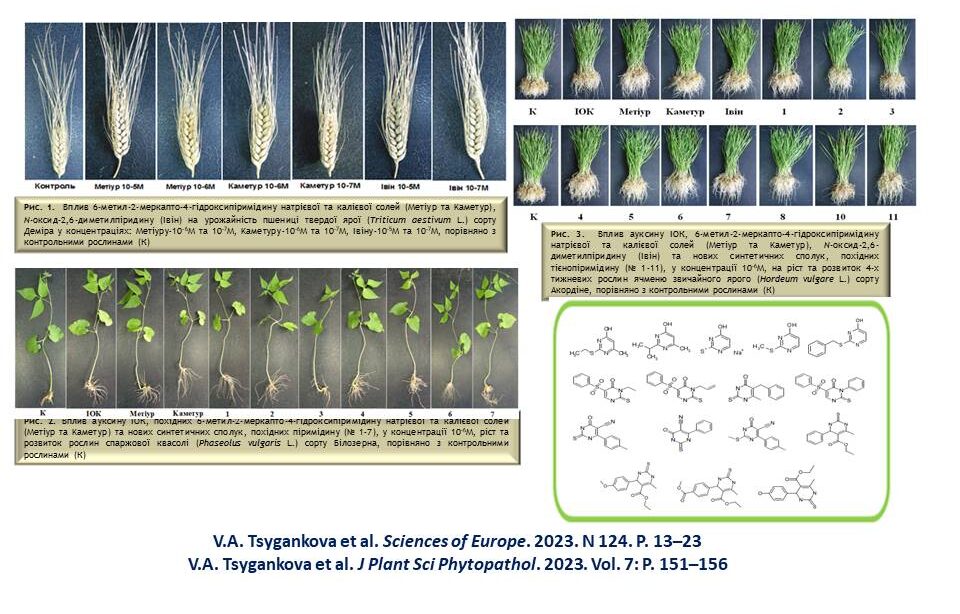
The influence of low molecular weight synthetic azaheterocyclic compounds on the growth and development of different plants, including wheat, barley, maise, sorghum, pea and soybean, during vegetation under normal conditions and abiotic stresses was investigated. The most active compounds were selected, and N-oxide-2,6-dimethylpyridine, sodium and potassium salts of 6-methyl-2-mercapto-4-hydroxypyrimidine, thioxopyrimidine and furopyrimidine derivatives were proposed for practical use in agriculture.
The most important scientific achievements of the Department in 2023
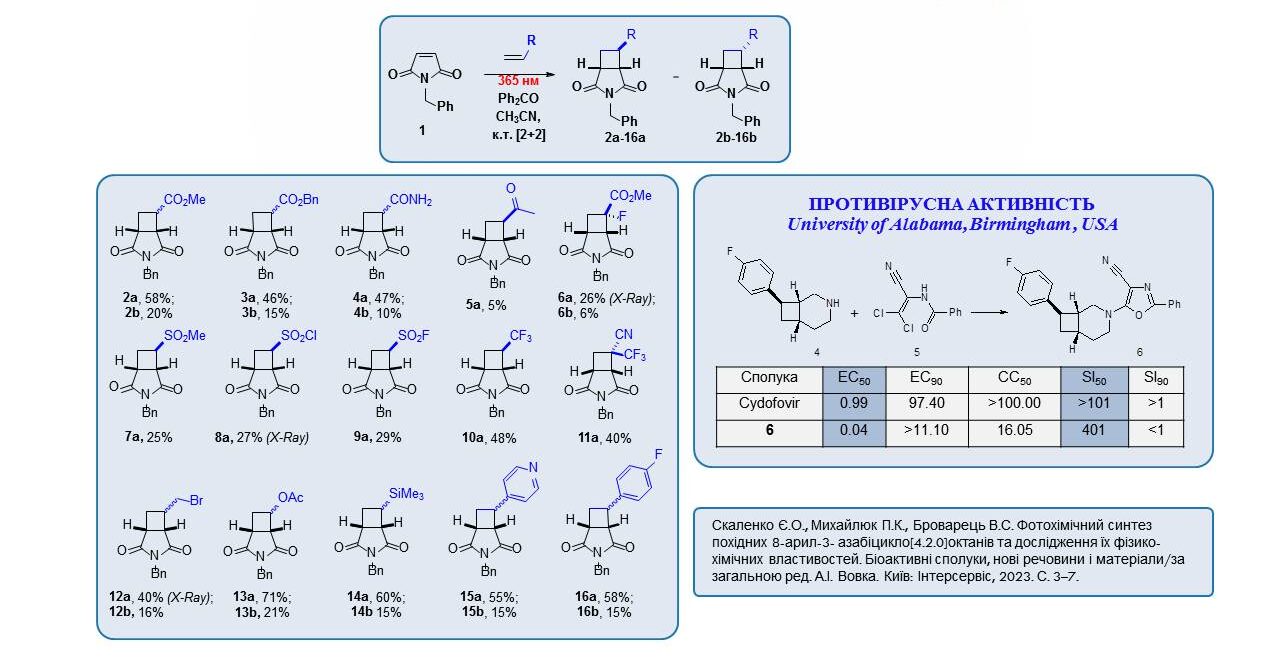
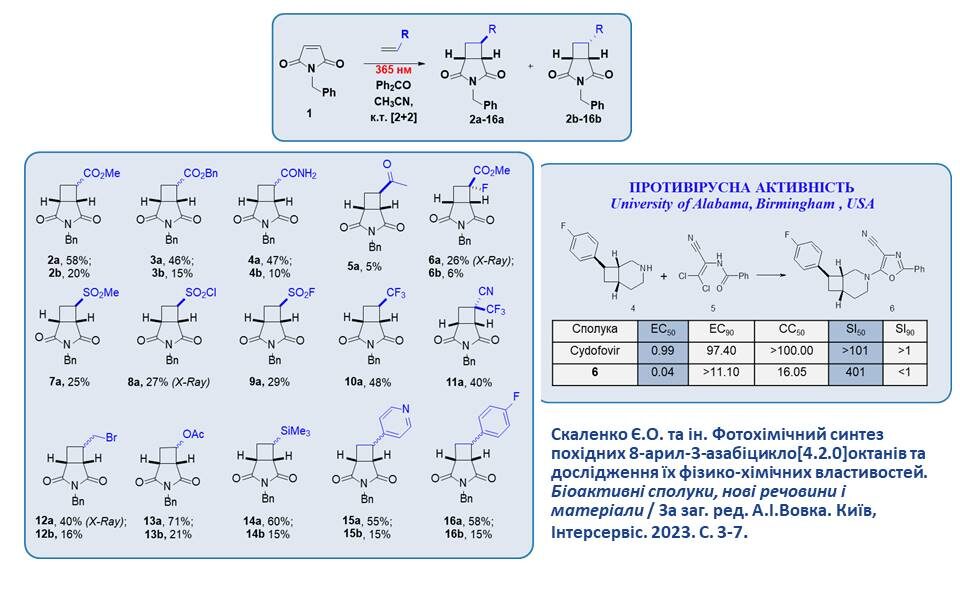
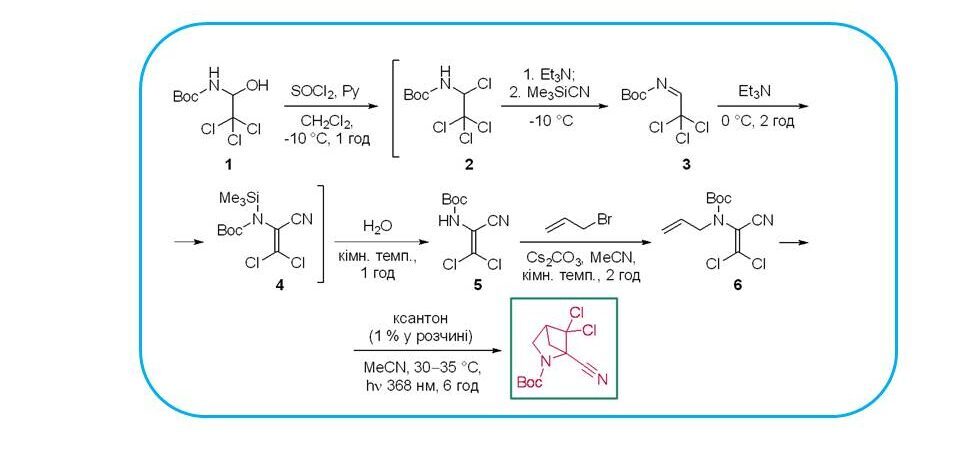
New derivatives of 3-azabicyclo[3.2.0]heptanes were synthesised by intermolecular heterocyclization at 365 nm. Compound 6, synthesised according to the scheme, showed significant activity against BK-virus. Compared to cidofovir, a well-known antiviral agent, compound 6 showed a 25-fold lower EC50 value and a 4-fold higher SI50 value. These data confirm the significant potential of azabicyclic compounds in future medicinal chemistry research.
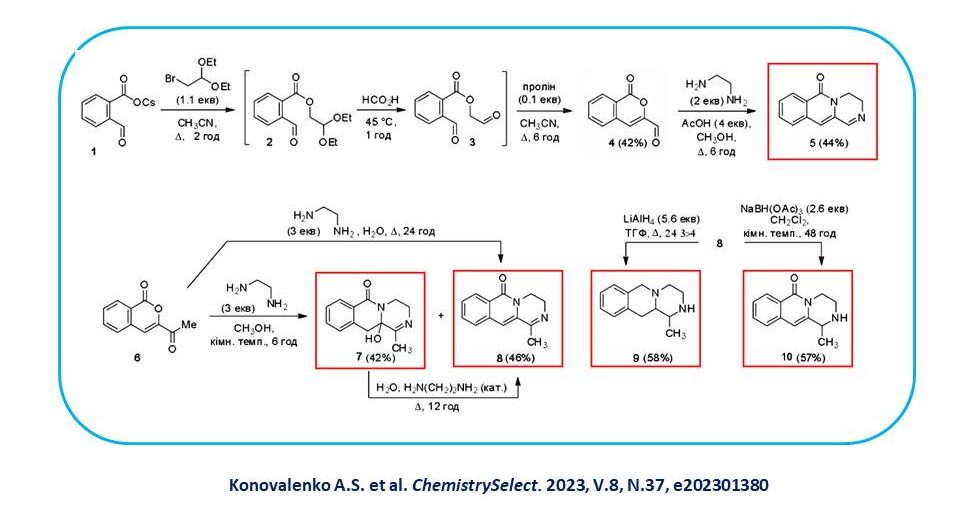
Based on 3-formyl and 3-acetylisocoumarin 1,6, new pyrazinoisoquinolones 5,7,8 were synthesised, which are interesting because of the possibility of their further transformations, in particular, reduction reactions 9,10 and the search for biologically active substances among them.
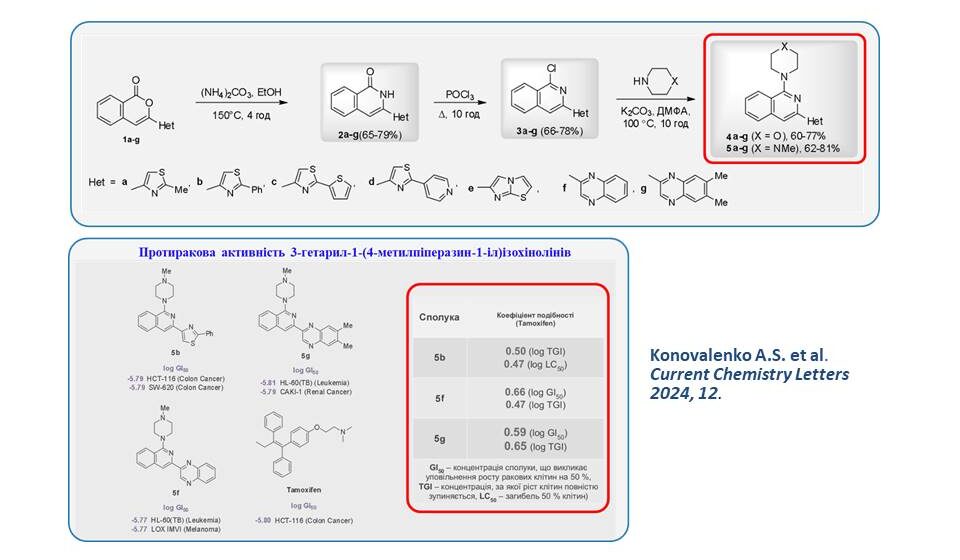
A strategy for the synthesis of substituted 1-aminoisoquinolines with heterocyclic substituents at position 3 has been developed, which consists of the conversion of isocoumarin 1 via the 1-2-3-4.5 transformation chain. The effect of the heterocyclic substituent on the anticancer activity of the investigated compounds was shown. According to the COMPARE analysis, the similarity of the activity profiles of the investigated compounds correlates most closely with that of the well-known anticancer drug Tamoxifen.
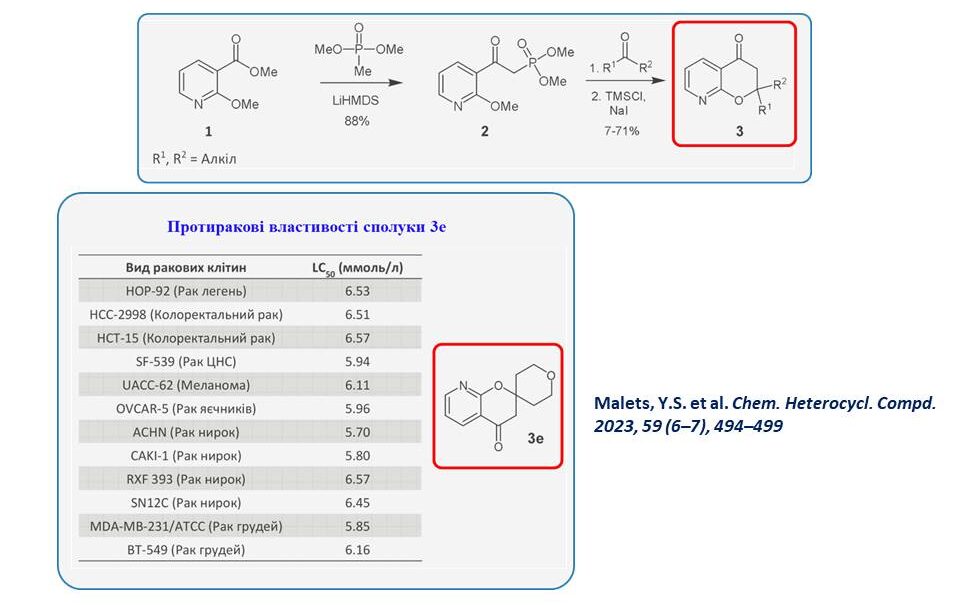
We synthesised 2-spiro-3-azachromanones and showed that compound 3 has significant antitumour potential in biological tests. In particular, an extended five-dose screening of compound 3e demonstrated millimolar activity against cancer cells. Thus, it can be argued that this is a ‘hit molecule’ that can become a drug candidate after the target has been identified.
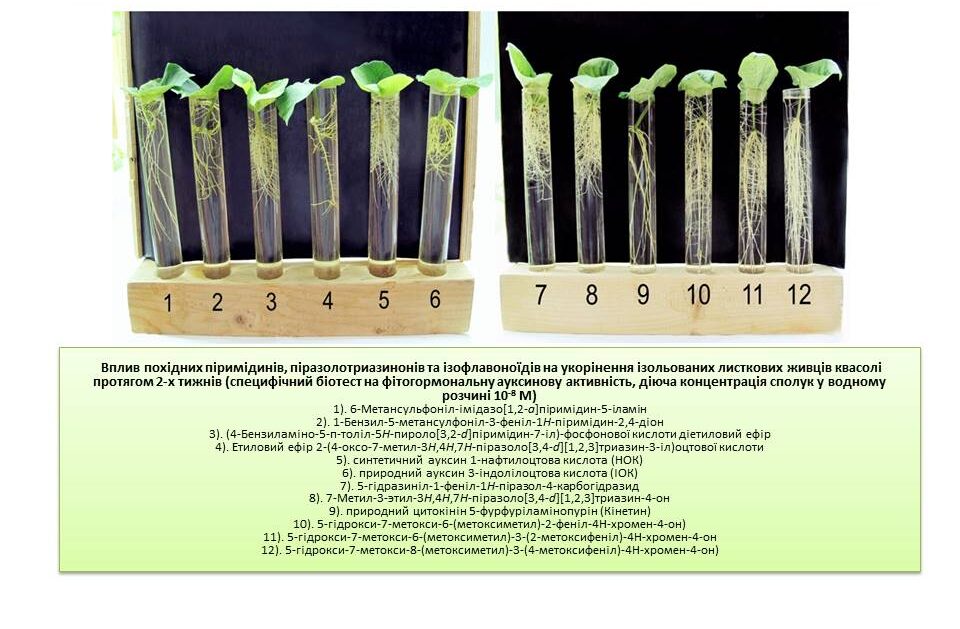
The effect of functionalised pyridine and pyrimidine derivatives on the growth and development of different varieties of wheat, barley, sorghum and bean plants during the growing season in the laboratory and on the yield of wheat plants in the field was investigated. The most active synthetic compounds with growth-regulating effects similar to phytohormones, auxins and cytokinins were selected by screening for morphometric and biochemical parameters of plants. Optimal physiologically active concentrations of low molecular weight azaheterocyclic compounds were selected, and the relationship between the chemical structure and biological activity of the studied synthetic compounds was analysed.
The most important scientific achievements of the Department in 2022
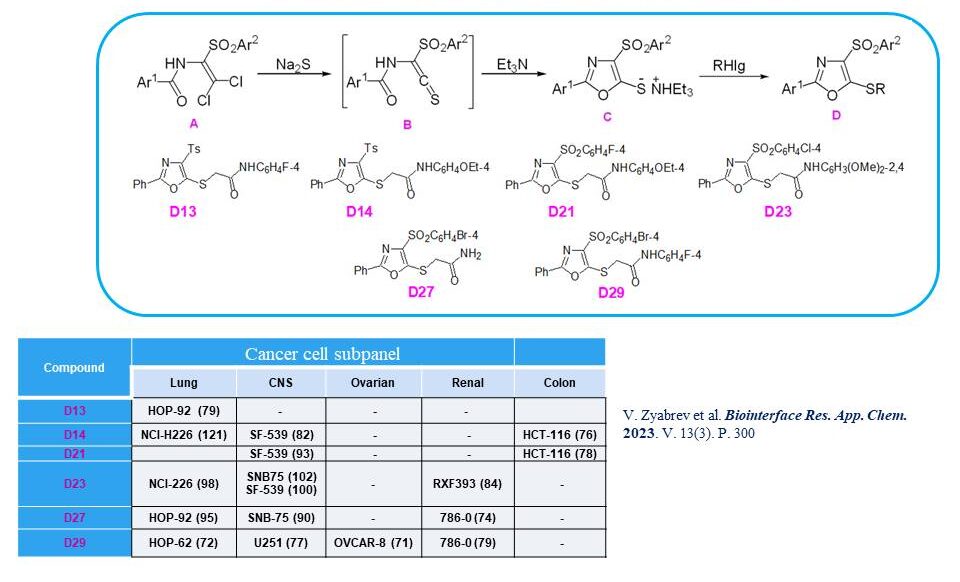
A new series of 4-arylsulfonyl-1,3-oxazoles was synthesised, and their anticancer activity was investigated. Among them, the most effective compounds were D14, D23 and D27, which demonstrated activity against various cancer cell lines, which makes it possible to consider them as candidates for further in-depth studies.
For the first time, 2-allylamino-3,3-dichloroacrylonitrile was used in a photoinitiated intramolecular [2+2]-cycloaddition, which led to the formation of N-Boc-protected 5,5-dichloro-2-azabicyclo[2.1.1]hexane-1-carbonitrile, a unique representative of 2-azabicyclo[2.1.1]hexanes with a nitrile group and a dichloromethylene fragment.
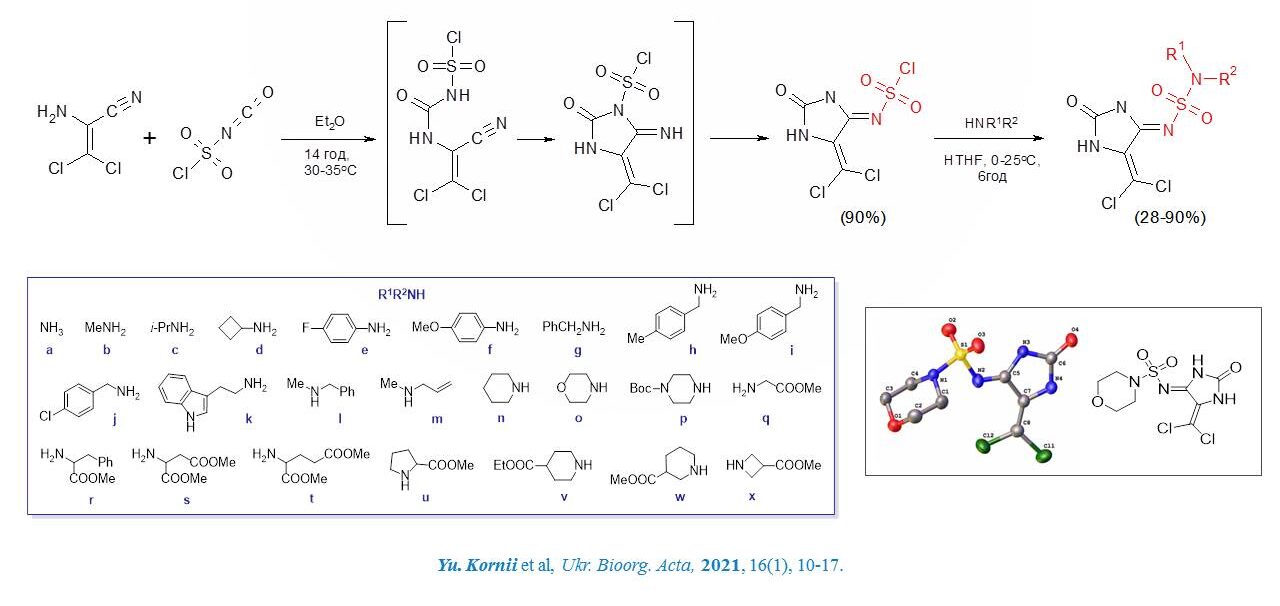
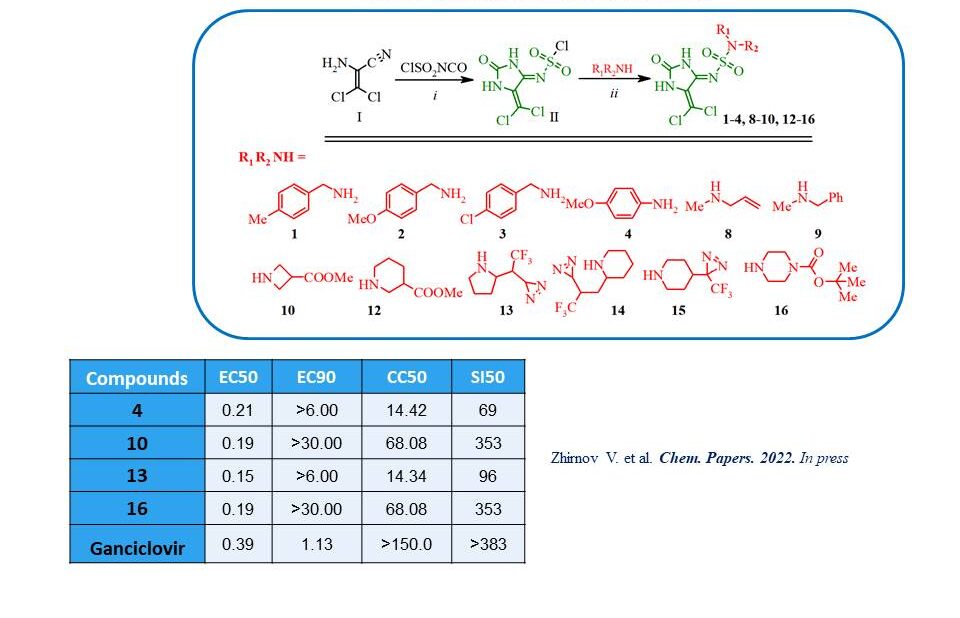
New 4-iminohydantoin sulfamide derivatives were synthesised by sequential action on aminodichloroacrylonitrile chlorosulfonyl isocyanate and amines. Their in vitro antiviral activity against human cytomegalovirus (HCMV) was studied. Biotests showed that the four compounds exhibited higher antiviral activity (EC50: 0.15–0.21 μM) against regular laboratory HCMV (strain AD-169) compared to that of ganciclovir (EC50: 0.11–0.39 μM).
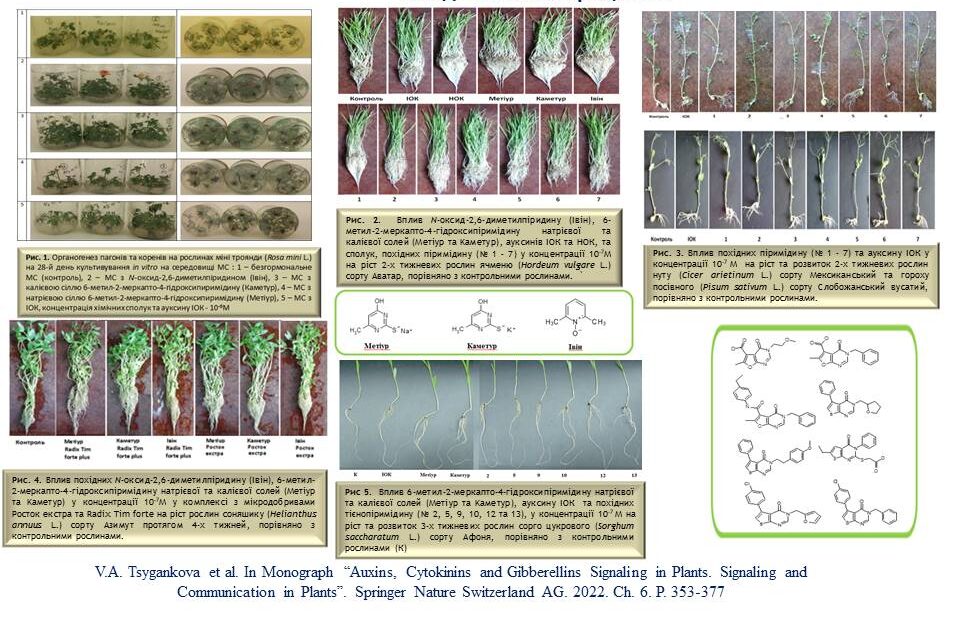
The influence of plant growth regulators based on N-oxide-2,6-dimethylpyridine, sodium and potassium salts of 6-methyl-2-mercapto-4-hydroxypyrimidine, furanopyrimidine and thienopyrimidine derivatives on the growth and development of plants (wheat, barley, sorghum, rye, sunflower, pea, chickpea, rape, flax and bean) during the growing season, as well as on the frequency of Agrobacterium-mediated transformation of tomato plants. The relationship between the chemical structure and biological activity of the studied synthetic compounds was analysed, which was species- and variety-specific. The practical use of the investigated compounds in agriculture to improve plant growth and in biotechnology to improve microclonal plant propagation is proposed.
The most important scientific achievements of the Department in 2021
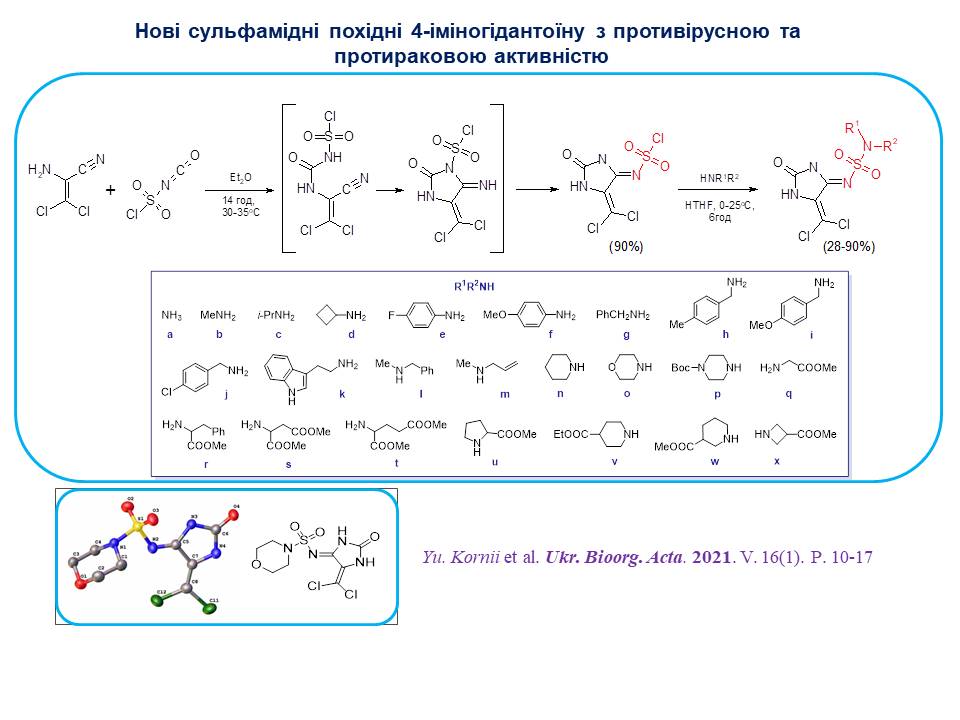
A convenient methodology for the synthesis of a new class of guantoin sulfamides by the interaction of aminodichloroacrylonitrile with chlorosulfonyl isocyanate has been developed. The study of antiviral and anticancer activity of the synthesised compounds convincingly demonstrates the decisive influence of substituents in the primary structure on the type and level of biological activity.
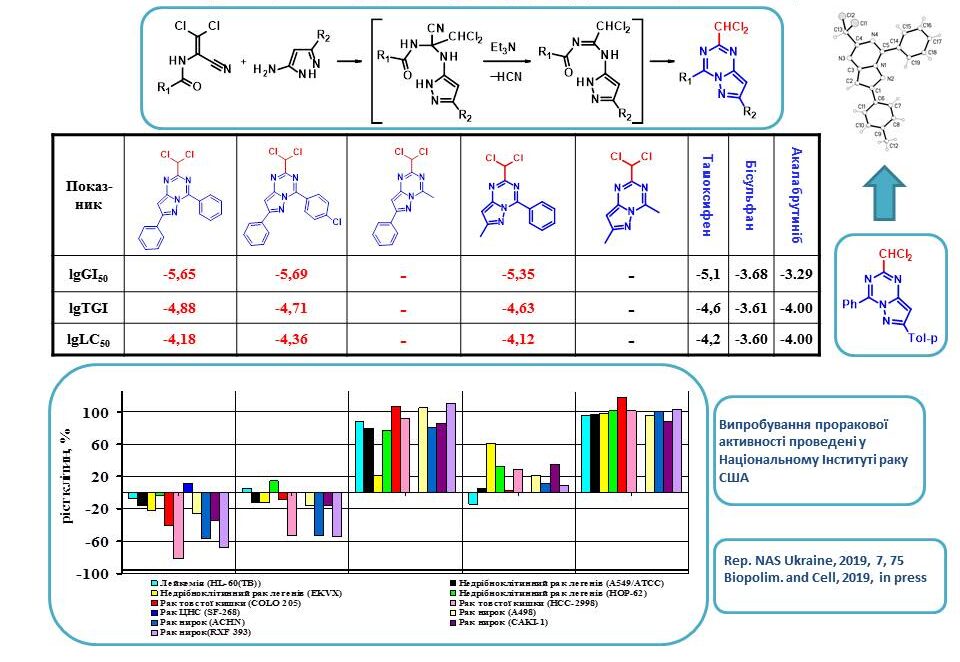
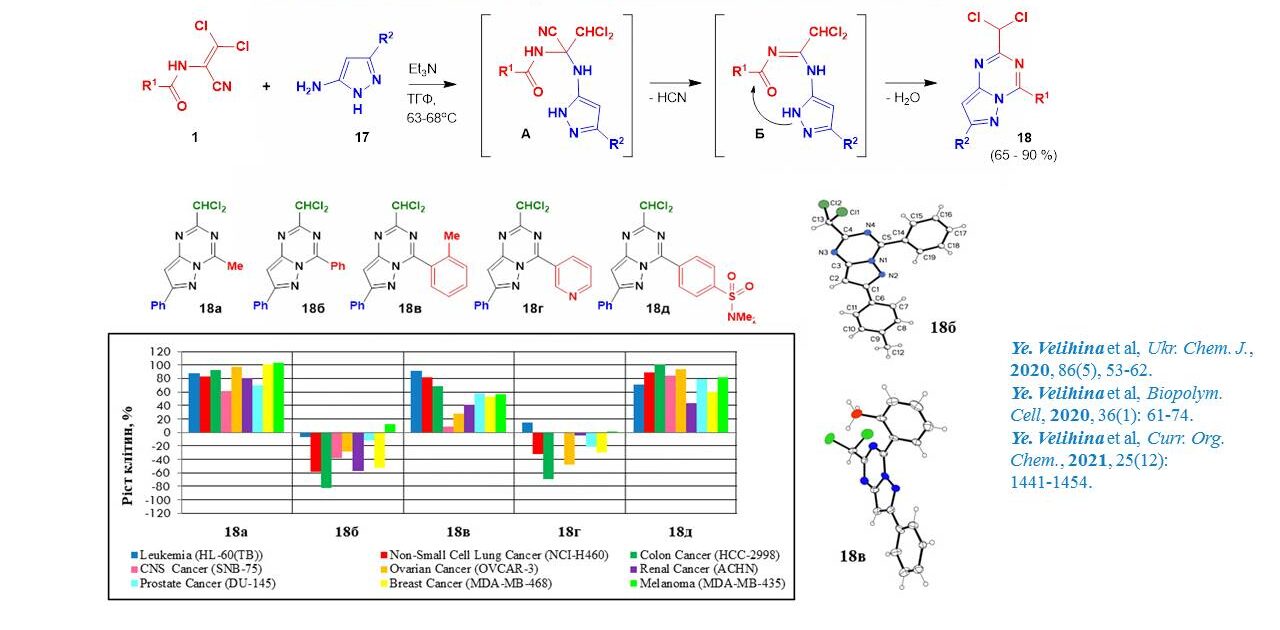
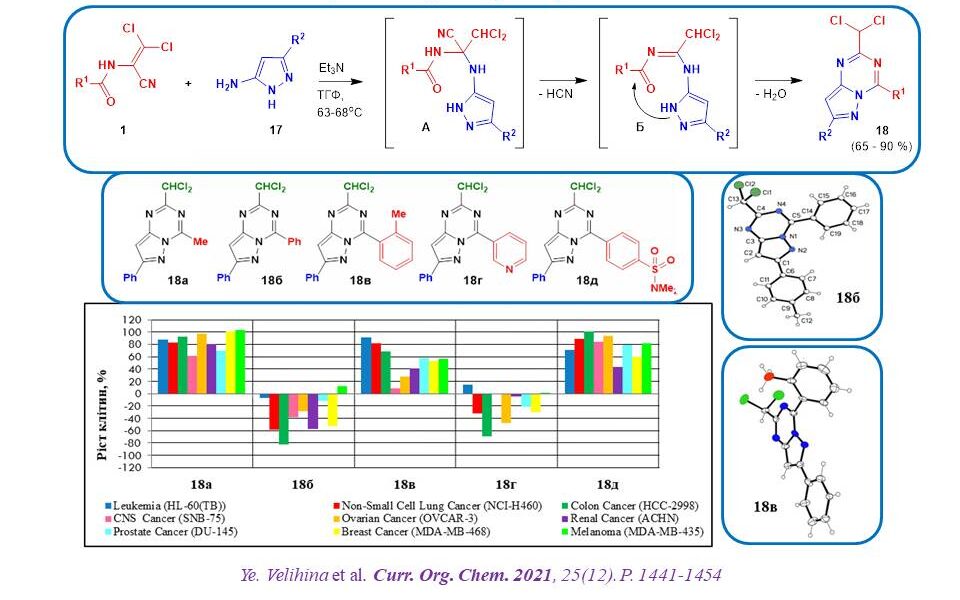
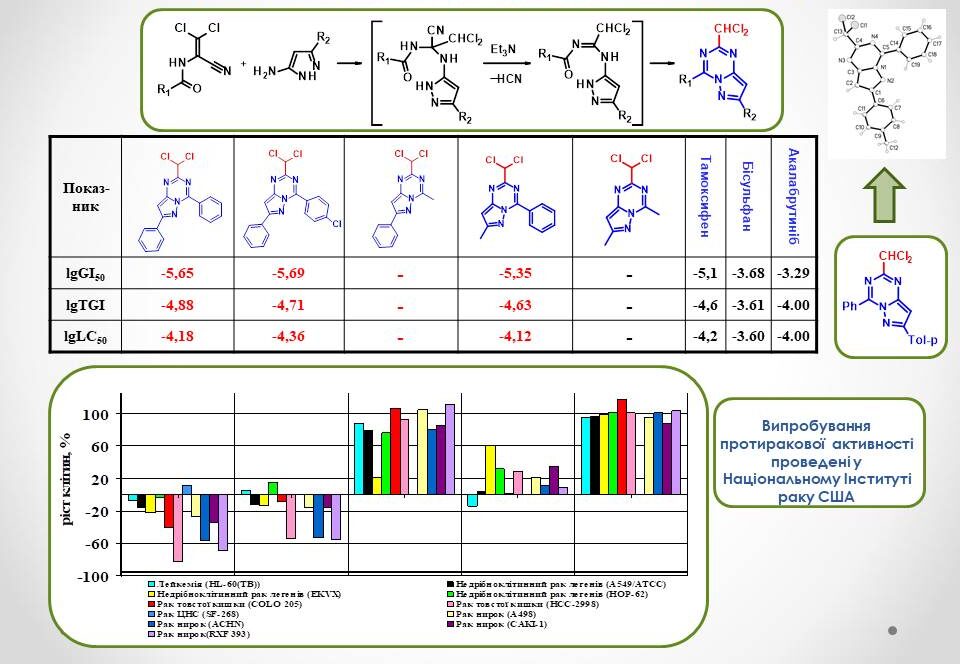
The interaction of dichloroacrylonitriles with 5-aminopyrazoles was used to synthesise new representatives of 2-chloromethylsubstituted pyrazolo[1,5-a]-[1,3,5]triazine derivatives. The study of their anticancer activity revealed that the nature of the substituent at position 4 plays a crucial role in the antitumour effect of these compounds. Thus, derivatives with an aryl substituent at C4 are extremely active. In addition, it is important that the aryl substituent and the pyrazolotriazine system are in the same plane, which was proved by PCA. The presence of a dichloromethyl group at position 4 of the bicyclic molecule is critical for the anticancer activity of these substances.
The most important scientific achievements of the Department in 2020
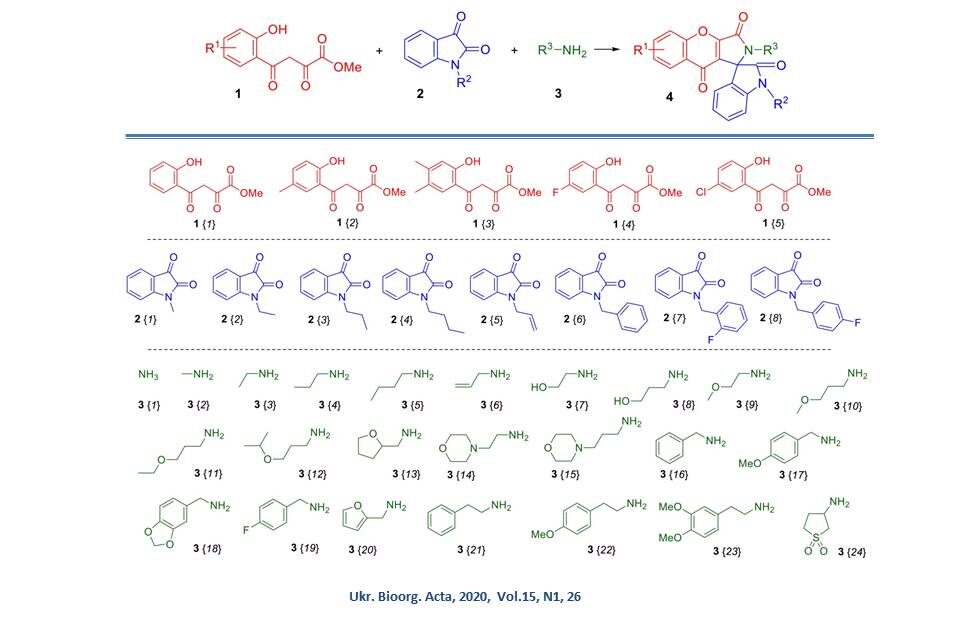
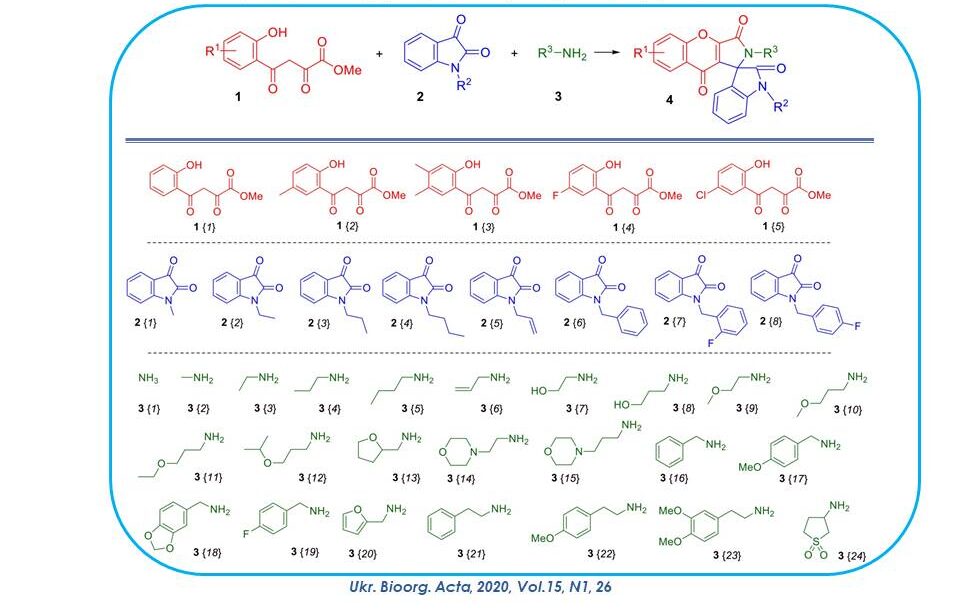
The three-component cyclisation of methyl esters of 4-(o-hydroxyphenyl)-2,4-dioxobutanoic acids of 1,N-substituted isatins 2 and primary amines 3 leading to the formation of 2H-spiro[chromeno[2,3-c]pyrrole-1,3'-indoline]-2',3,9-trions, fragments of which are widely represented among natural bioactive compounds, in particular, in the composition of alkaloids, has been investigated. It was found that this three-component heterocyclization is a versatile and efficient tool for the synthesis of the abovementioned heterocyclic system. Based on dioxobutanoic acid esters (highlighted in red on the slide), isatins with alkyl, allyl and benzyl substituents at position 1 (blue), as well as a wide range of different aliphatic amines (green), a combinatorial library of 122 derivatives was created in yields exceeding 70%.
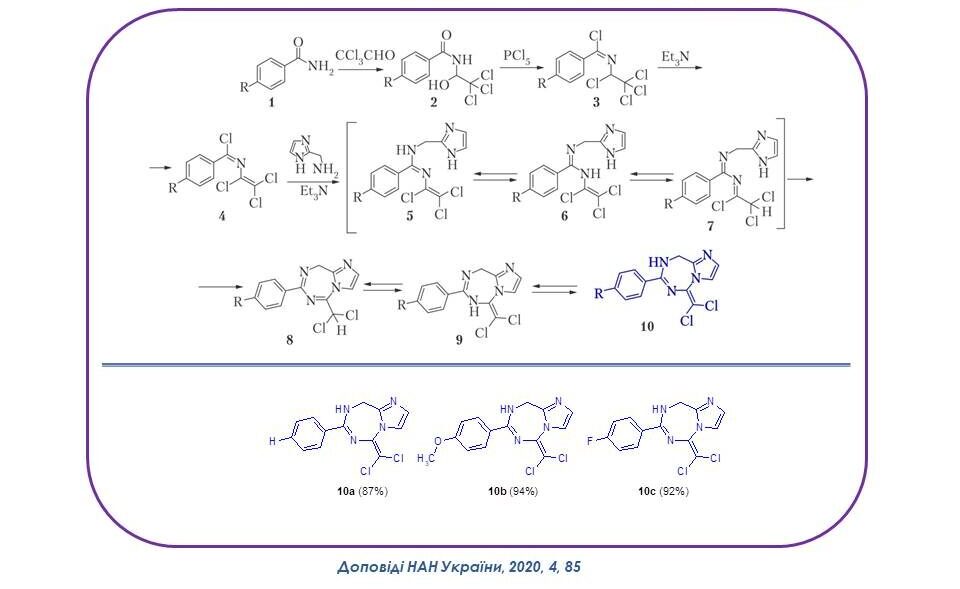
It has been shown that the interaction of 1-aryl-2-azatetrachloro-1,3-butadiene with 2-(aminomethyl)imidazole occurs by regioselective anelation to the imidazole ring of the triazepine system with the formation of the first representatives of the new heterocyclic system 5H-imidazo[1,2-e][1,3,5]triazepine in high yields, which contain two pharmacophore fragments, namely, imidazole and triazepine. Their formation occurs through a number of intermediates and prototropic forms, which are shown in the scheme.
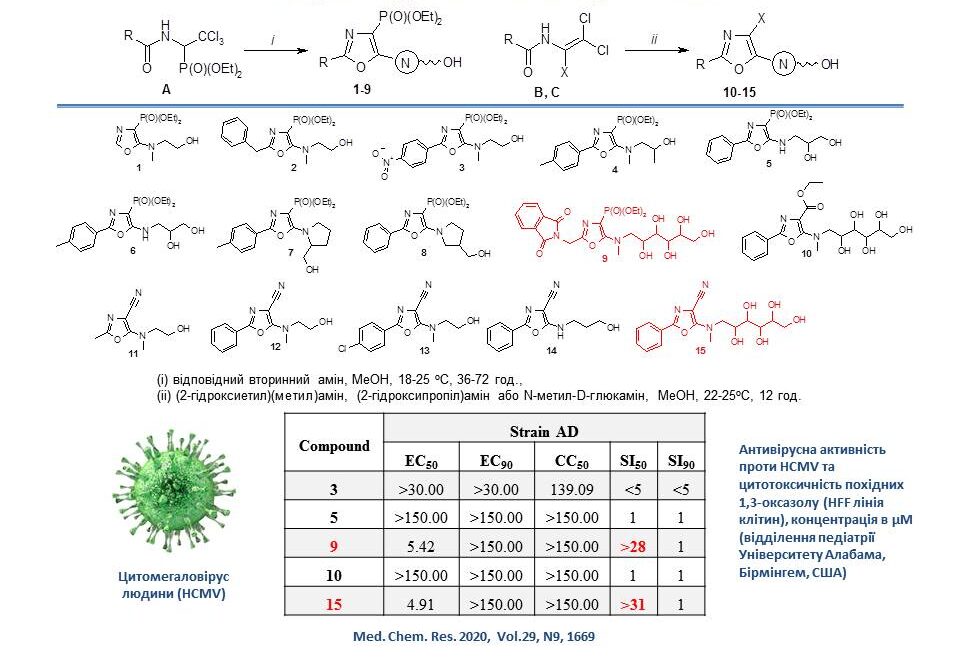
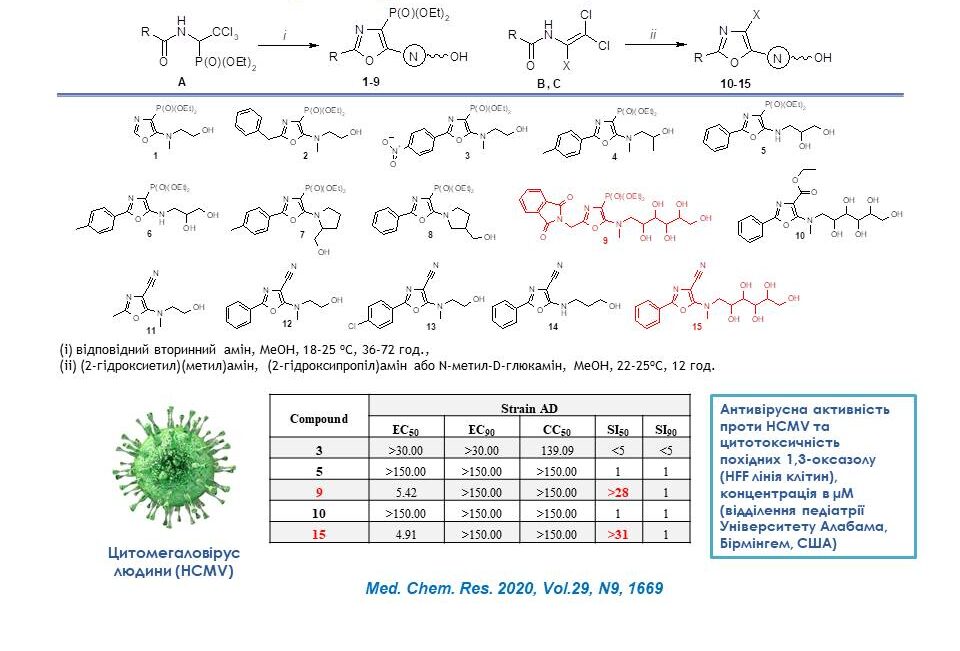
Several new substituted 5-hydroxyalkylamino-1,3-oxazoles were synthesised to investigate their antiviral activity and cytotoxicity in vitro. They were tested for activity against human cytomegalovirus (HCMV). The test results showed that compounds 9 and 15 exhibited moderate antiviral activity against a regular laboratory strain of HCMV (AD169) in HFF cells. Further functional modifications of these compounds may lead to the development of new 5-hydroxyalkylamino-1,3-oxazole derivatives with higher anti-HCMV activity.
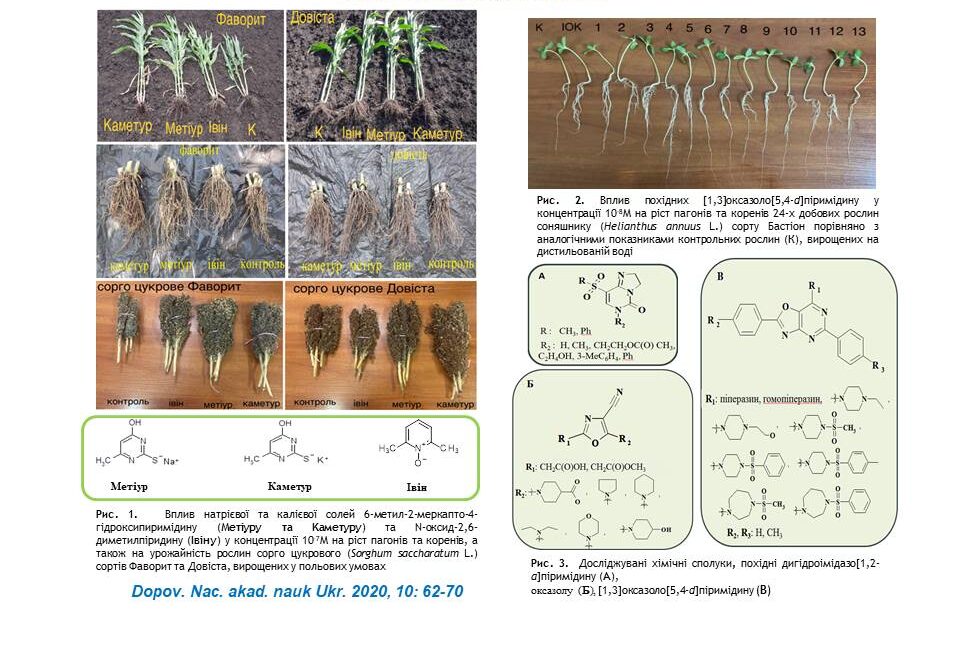
The influence of sodium and potassium salts of 6-methyl-2-mercapto-4-hydroxypyrimidine (Metiuru and Kameturu), N-oxide-2,6-dimethylpyridine (Ivinu), imidazo[1,2-a]pyrimidine, oxazole and [1,3]oxazolo[5,4-d]pyrimidine on the growth and development of different plant varieties of barley, chickpea, sunflower and sorghum has been researched. It was found that the treatment of plant seeds with aqueous solutions of these compounds at micromolar and submicromolar concentrations significantly improves physiological parameters (plant height, length and number of roots), biochemical parameters (content of photosynthetic pigments) and increases plant yields (plant biomass, panicle length and grain weight). The practical use of synthetic compounds for the pre-sowing treatment of seeds of these crops to improve their growth and increase productivity is proposed.
The most important scientific achievements of the Department in 2019
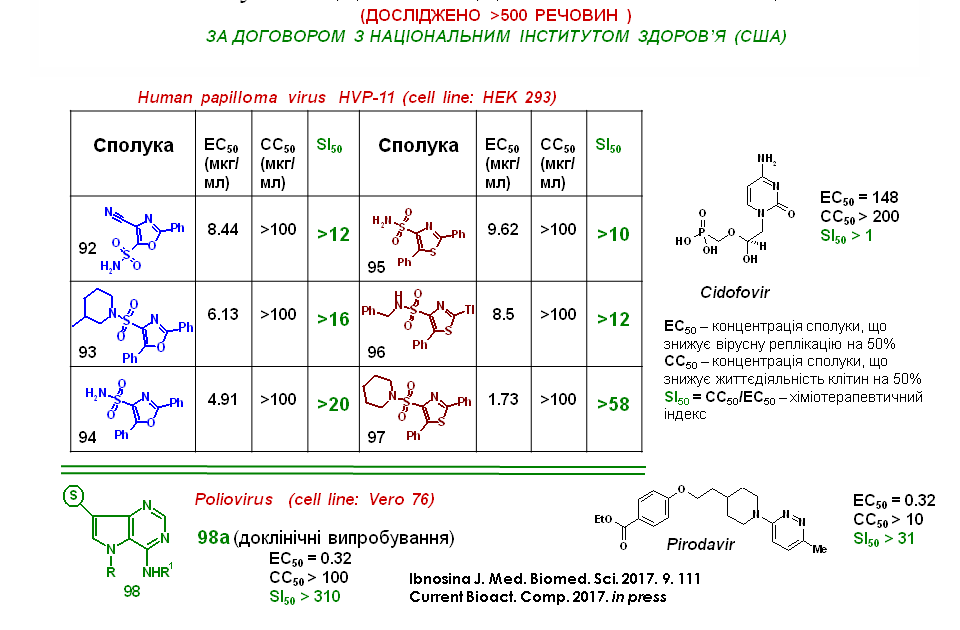
Based on sulfonyl-substituted aminoacrylonitriles 1 and halogenated halides, the synthesis of condensed pyrimidine derivatives, imidazo[1,2-c]pyrimidine and pyrimido[1,6-a]pyrimidine, whose structure has been reliably proved by X-ray crystallography analysis, was developed. The synthesised compounds were active against HPV and papillomavirus, with quantitative parameters close to or significantly higher than those of the reference drug Cidofovir.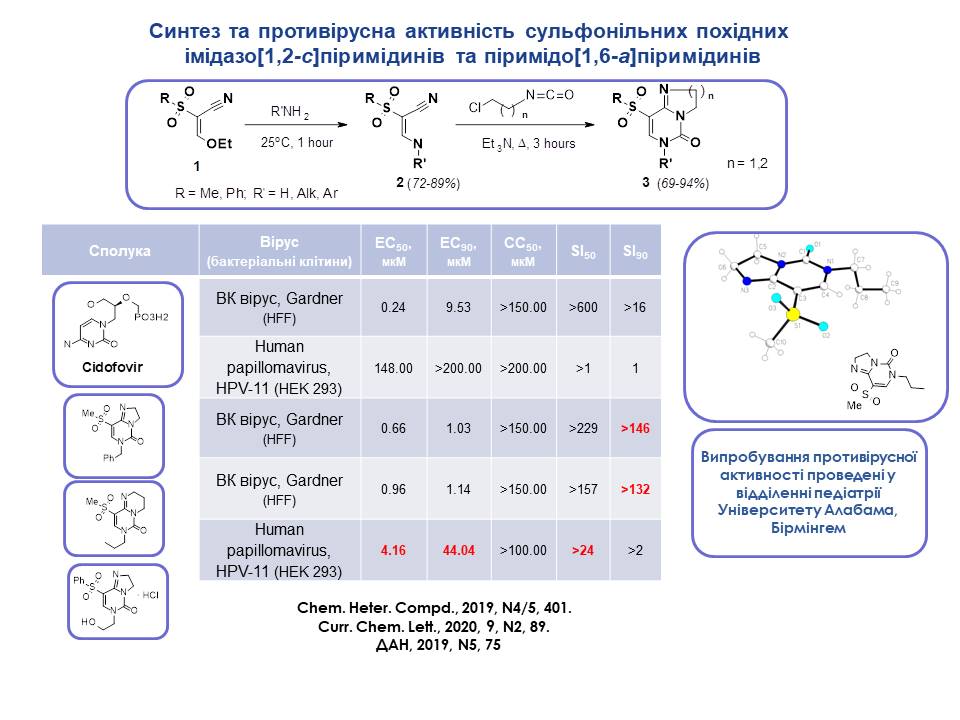
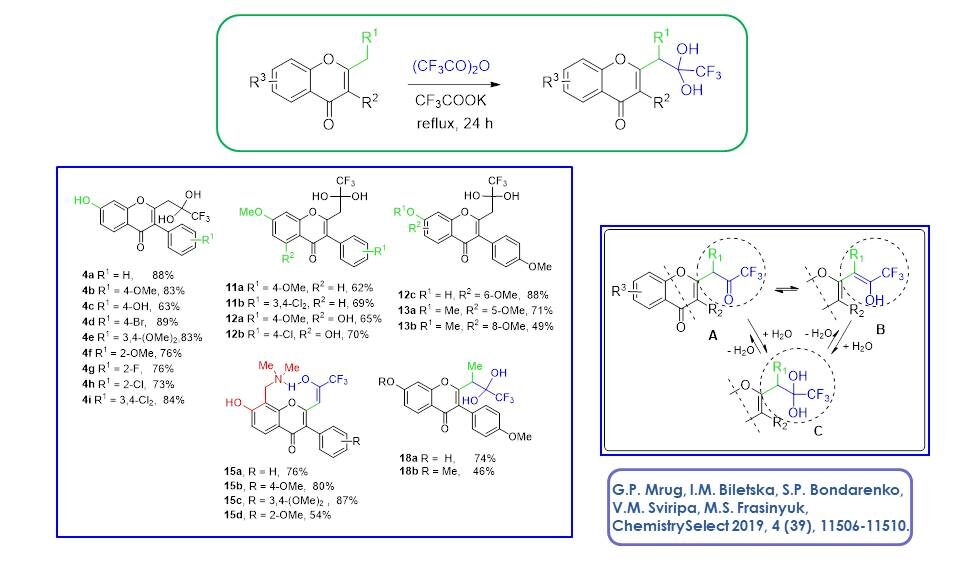
An efficient method for the preparation of hydroxylated homoisoflavonoids and 3-heterarylmethylchromones, analogues of natural compounds, has been developed. This approach avoids multistage synthesis and does not require protection of hydroxyl groups.

The most important scientific achievements of the Department in 2018
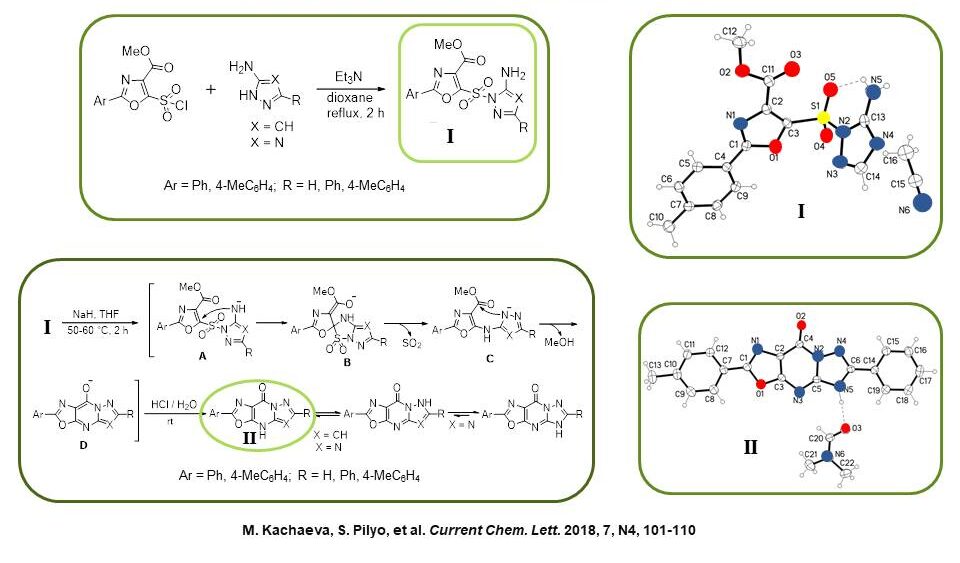
It has been shown that the interaction of 2-aryl-5-(chlorosulfonyl)-1,3-oxazole-4-carboxylates with aminoazoles is regioselective on the endocyclic nitrogen atom. Further action on the formation of sodium hydride products via the Smiles rearrangement yielded previously unknown derivatives of oxazolopyrazolopyrimidine and oxazolotriazolopyrimidine.
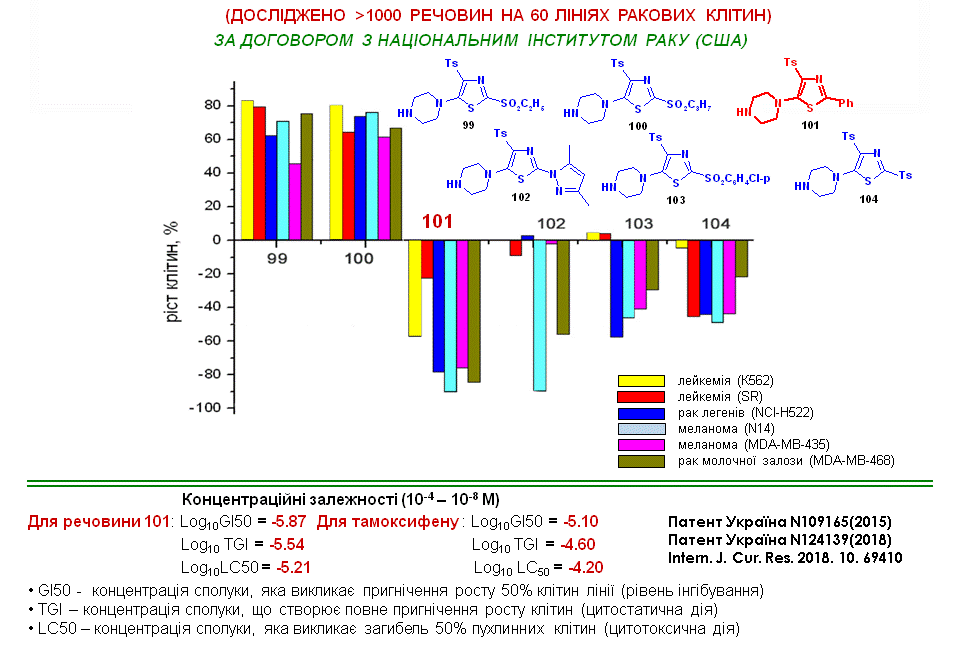
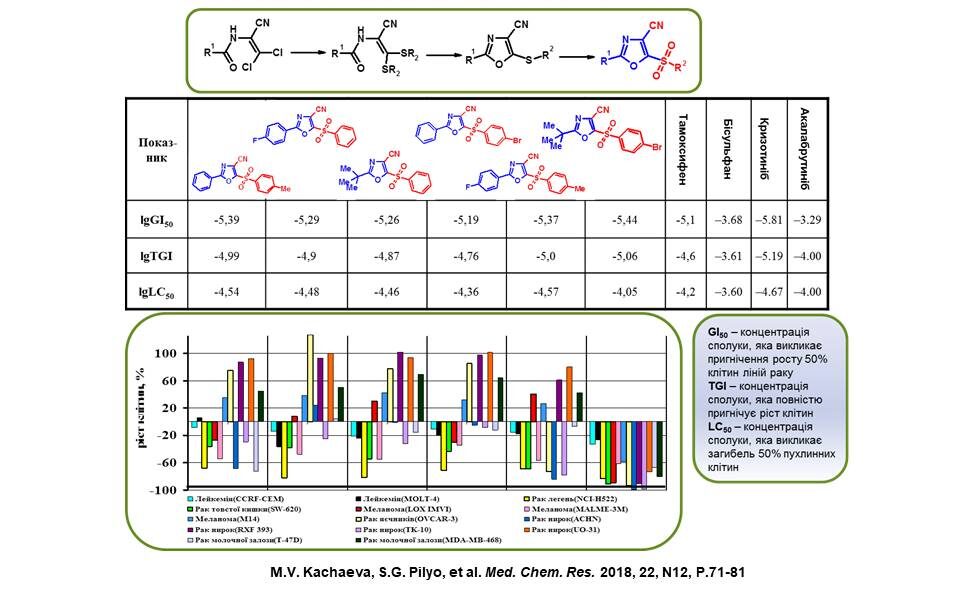
New oxazole derivatives containing a nitrile group at position 4 and an arylsulfonyl group at position 5 were synthesised. Among them, ‘leading compounds’ were identified that have significant antitumour activity against the cancer cell lines presented on the slide and exceed the performance of known antitumour drugs, Tamoxifen, Bisulfan, Crizotinib and Acalabrutinib.
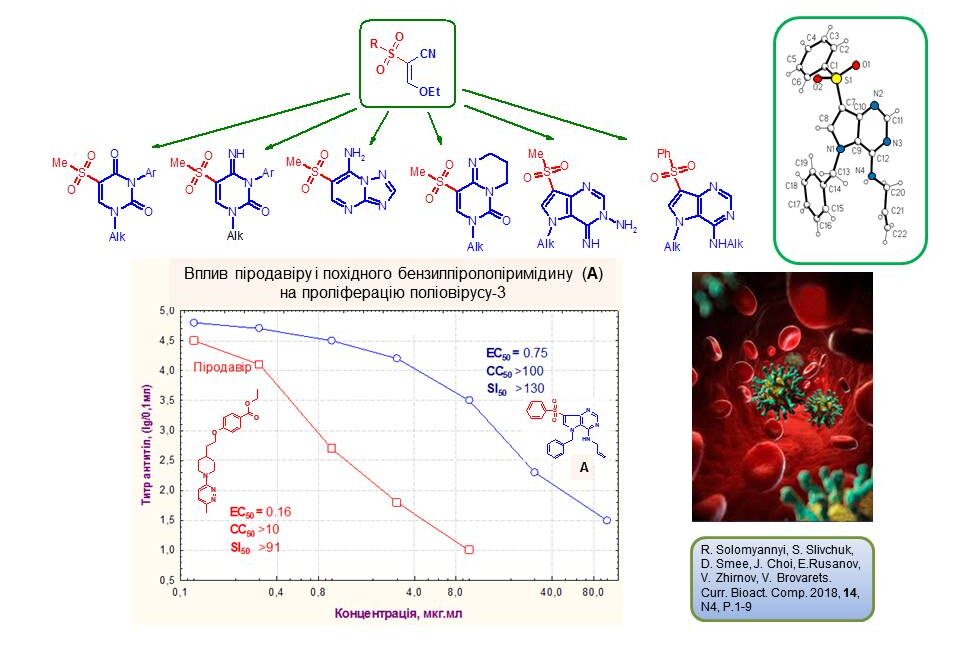
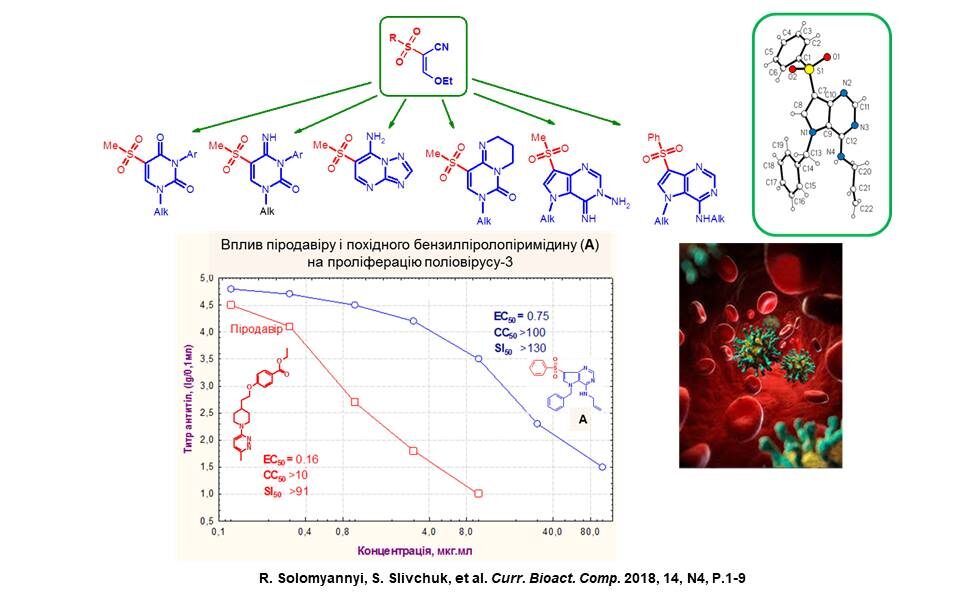
New pyrimidines and their condensed derivatives were synthesised based on available ethoxyacrylonitriles. The new products are non-toxic compounds with inhibitory effects against poliovirus-3 in vitro. The most active among them, compound A, inhibits viral proliferation and cytopathic activity with lower efficiency than Pirodavir, but has a higher selectivity index, indicating the prospects of searching for antiviral compounds among the investigated pyrimidine derivatives.
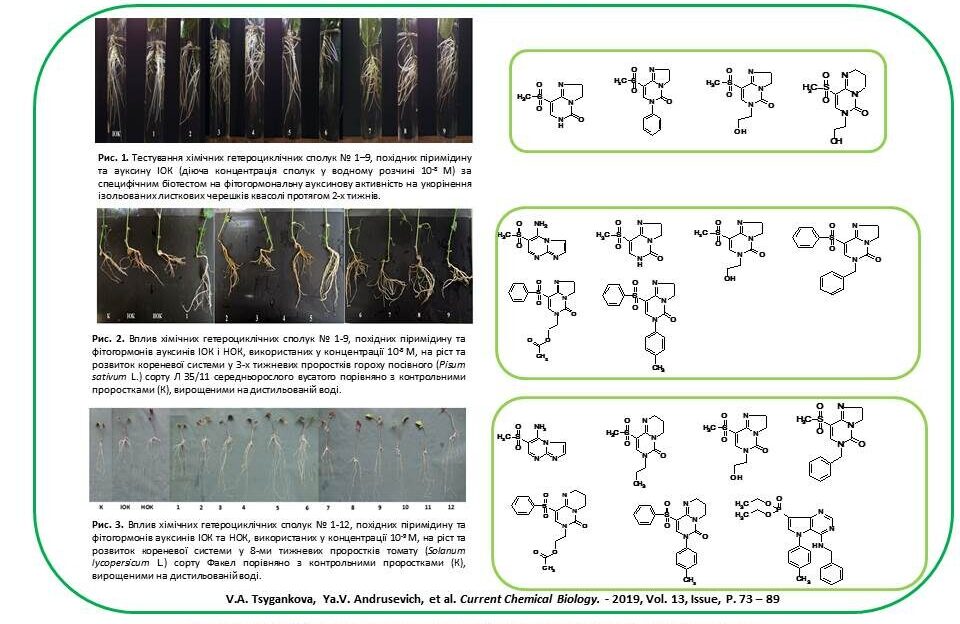
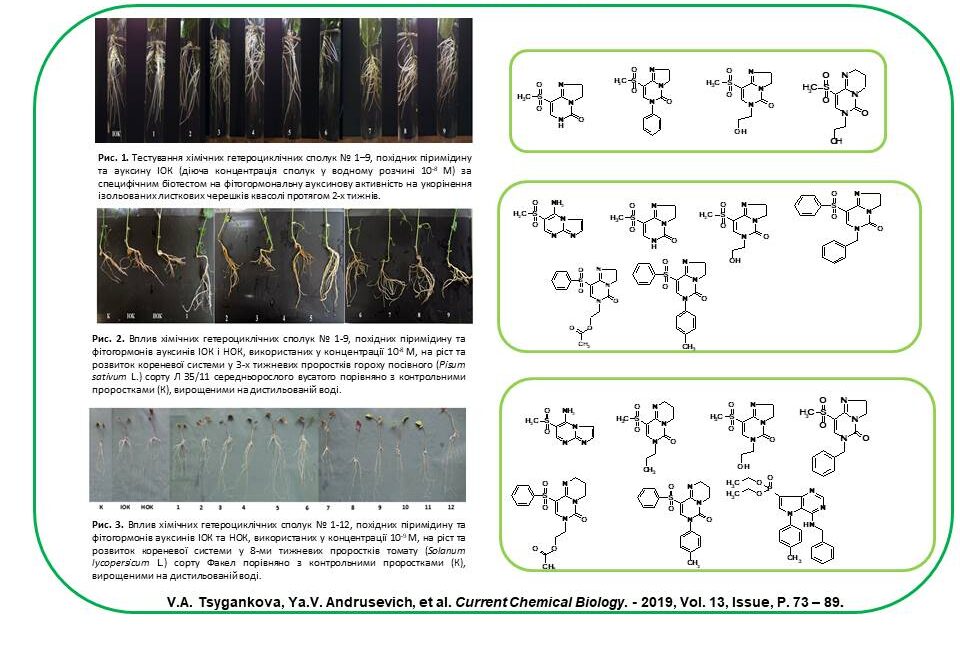
New plant growth regulators (for bean, tomato, pea, pepper, corn, wheat, radish and carrot) were developed based on synthetic low molecular weight heterocyclic compounds derived from pyrimidine and pyrazole. It has been found that the growth-regulating activity of synthetic compounds used in concentrations of 10–7–10–9 M is equal to or exceeds the activity of phytohormones auxins IOC and NOC, explained by the stimulating effect of these compounds on the processes of cell elongation, proliferation and differentiation, activation of photosynthetic processes and protein synthesis, as well as an increase in the activity of the catalase enzyme in plant cells. These processes accelerate and improve plant growth and development.
The most important scientific achievements of the Department in 2017
A method for the synthesis of 6- and 8-hydroxy(alkoxy)methyl derivatives of 7-hydroxyflavonoids with high antiproliferative activity has been developed. The use of 8-substituted 7-hydroxyflavonoids in the Diels-Alder hetero-reaction for the modification of flavonoids was proposed.
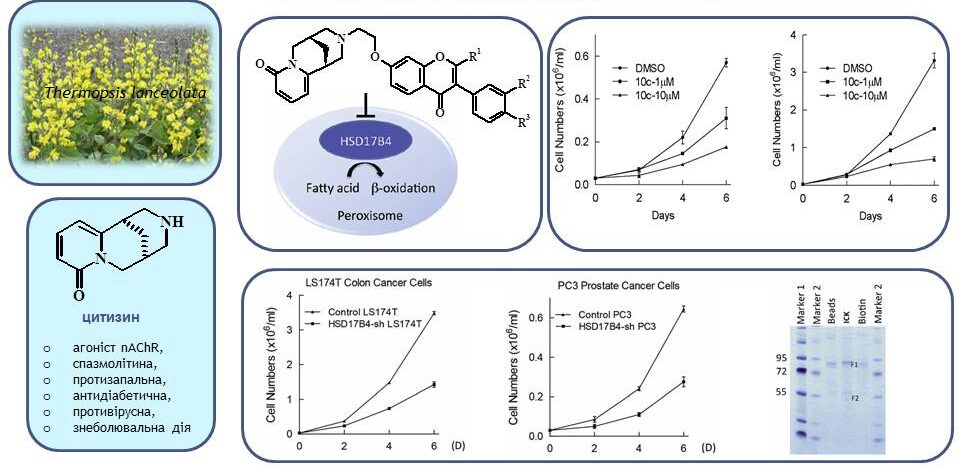
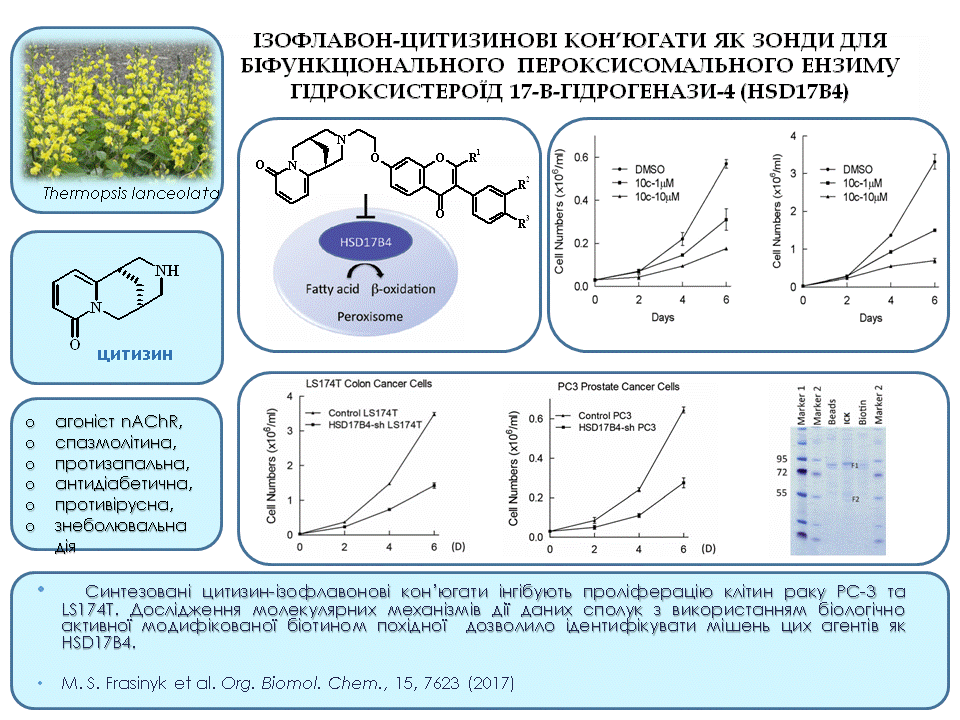
To date, numerous derivatives of the natural alkaloid cytisine with various heterocyclic fragments have been obtained: coumarin, 1,2,3-triazole, 1,2,4-thiadiazole, 1,3,4-thiadiazole. These compounds have a wide range of biological activity. For the first time, we have synthesised functional cytisine derivatives (4–6) that contain the pharmacophore 1,3-oxazole fragment. The available 1-acylamino-2,2-dichloroacrylonitrile (1) and related phosphorus-containing reagents (2,3) were used as starting materials. Compounds (4–6) are promising for further research of anti-inflammatory, antimicrobial, and insecticidal agents.
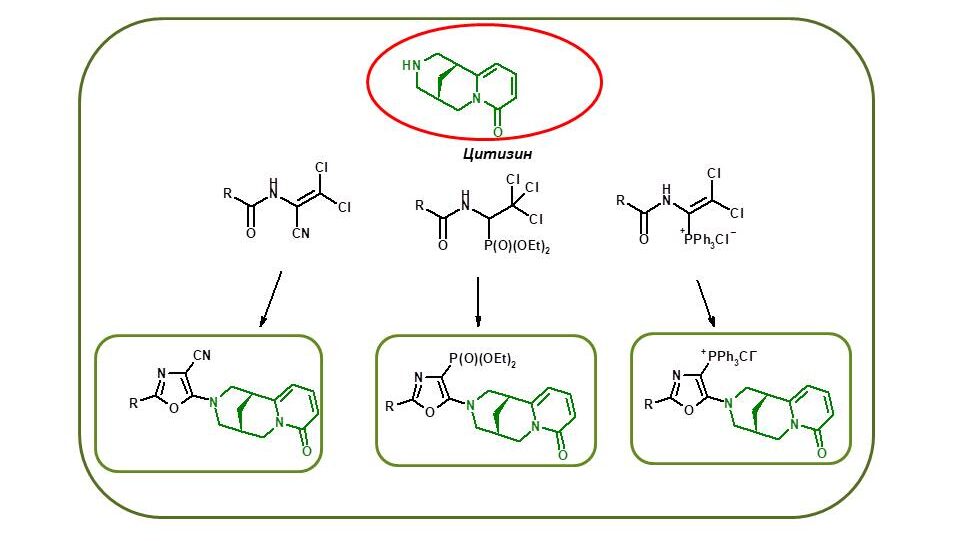
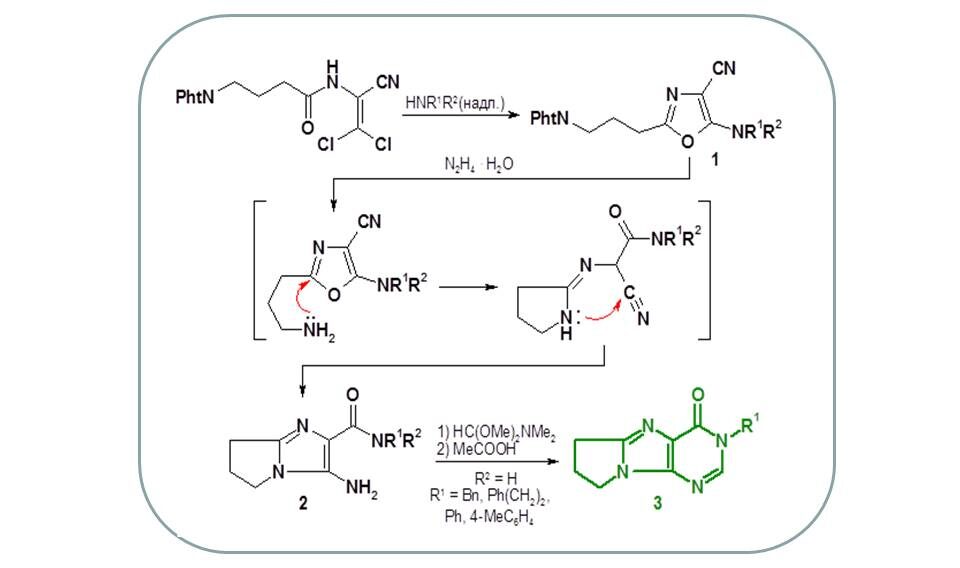
For the first time, it was shown that the hydrazinolysis of 5-amino-4-cyano-1,3-oxazole derivatives (1) containing a phthalimidopropyl fragment at position 2 undergoes recyclisation, leading to the formation of new hydrogenated 3-aminopyrrolo[1,2-a]imidazole-2-carboxamides (2). The latter were used for the synthesis of pyrrolopurinones [3], analogues of known biologically active substances [4–6].
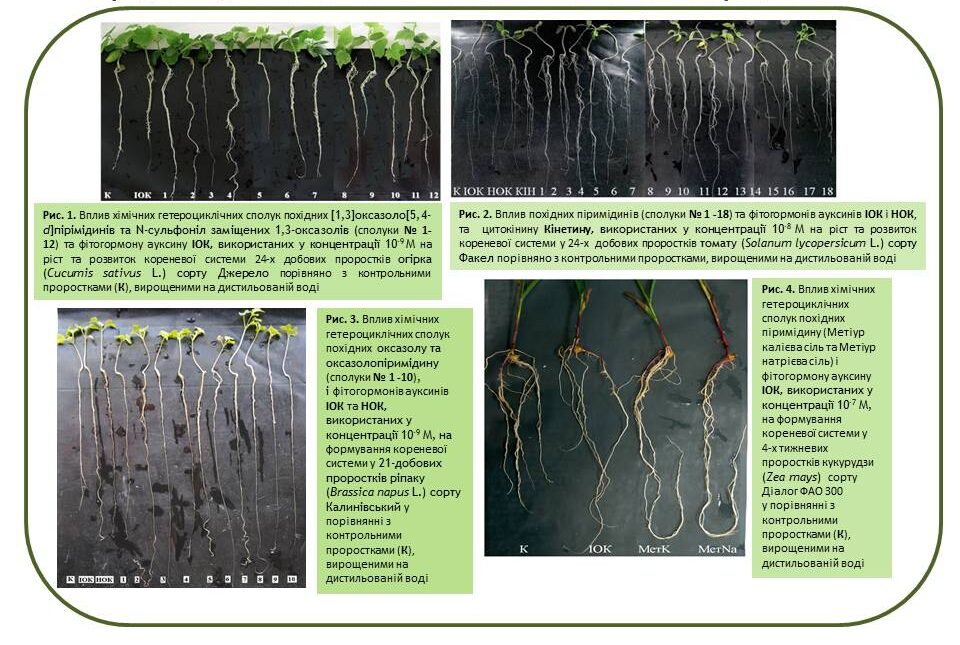
Based on synthetic five- and six-membered low-molecular-weight heterocyclic compounds of the pyrimidine, pyrazolotriazine, oxazole, oxazolo-pyrimidine and N-sulfonyl substituted oxazole derivatives, new effective environmentally safe regulators for accelerating and improving the growth and development of plants (cucumbers, tomatoes, corn, peas, barley, wheat, flax and rape) were developed. It was found that the activity of these compounds is similar to or exceeds the activity of natural and synthetic growth regulators (IAA, NAA, Kinetin).
The most important scientific achievements of the Department in 2016
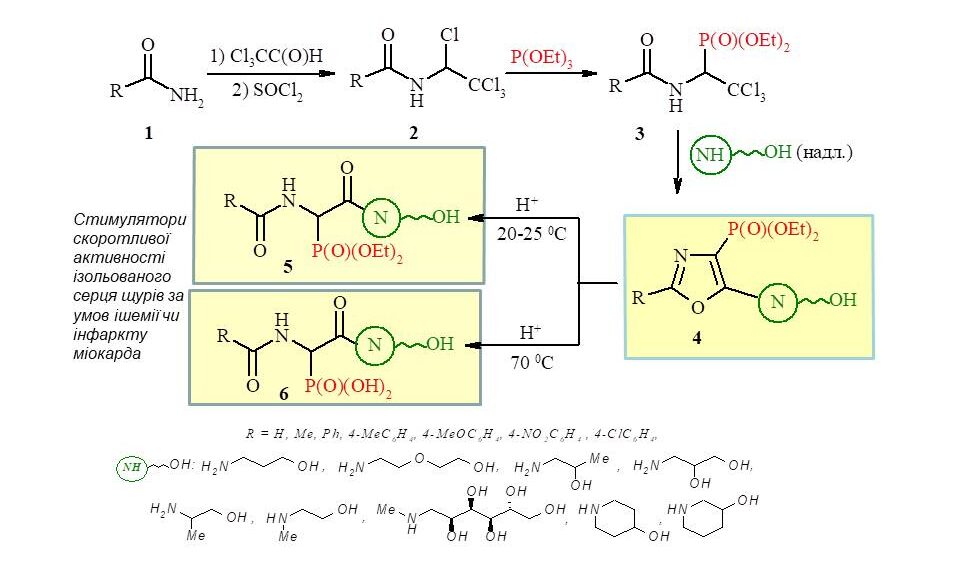
From available diethyl esters of 1-acylamino-2,2,2-trichloroethylphosphonic acids (3), new derivatives of 1,3-oxazole-4-ylphosphonic acids (4) containing residues of various pharmacophore amino alcohols at position 5 of the oxazole ring were synthesised. The latter was used for the synthesis of new phosphorylated peptidomimetics (5,6) containing alcohol residues and being potential biologically active substances.
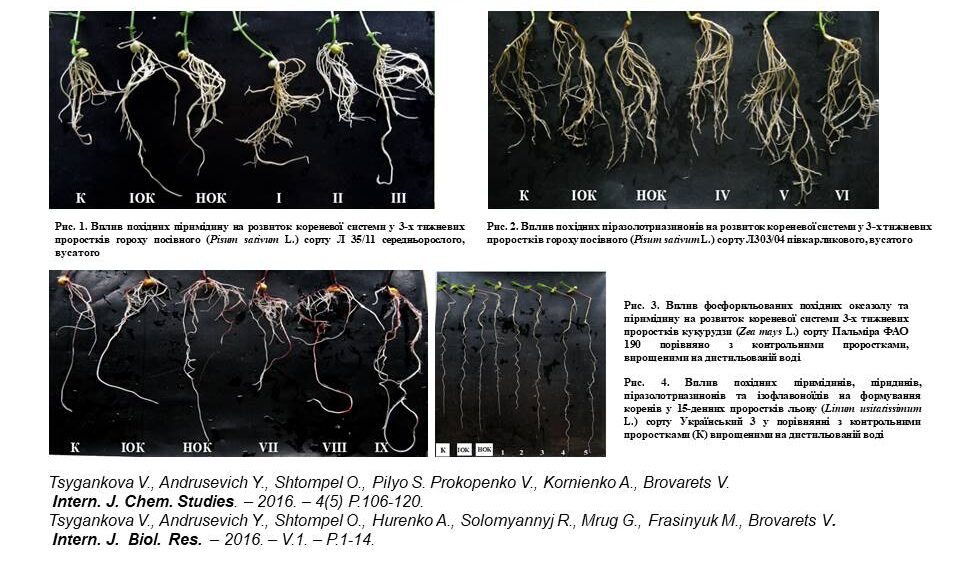
Based on synthetic five- and six-membered low-molecular-weight heterocyclic compounds (derivatives of pyridines, pyrimidines, pyrazolotriazinones, isoflavonoids, oxazoles oxazolo-pyrimidines and N-sulfonyl substituted oxazoles) new effective environmentally safe regulators for accelerating and improving the growth and development of plants (peas, corn, tomatoes, cucumbers, flax and pumpkin), which show activity equal to or exceeding that of natural and synthetic growth regulators (IAA, NAA, Kinetin).
The most important scientific achievements of the Department in 2015
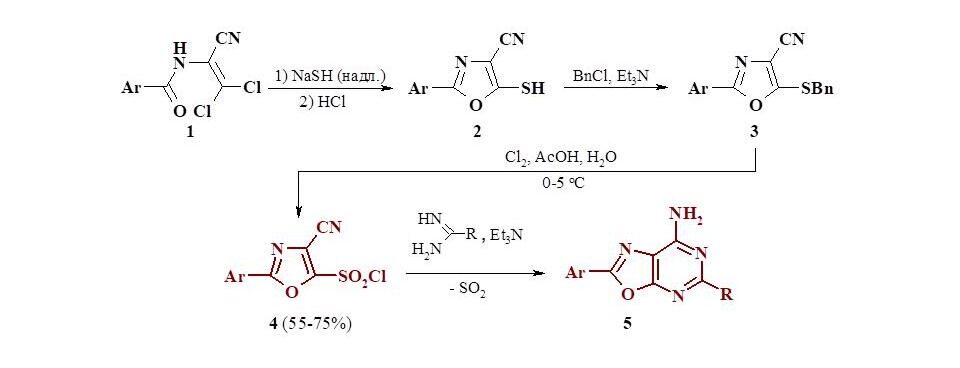
A preparative method for the synthesis of 1,3-oxazole-5-sulfonyl chlorides containing a nitrile group at position 4 was developed. For this purpose, available dichloroacrylonitriles (1) were used, which were first converted into 5-mercapto derivatives of oxazoles (2) and (3). Chlorination of the latter gave the corresponding sulfonyl chlorides (4) in high yields. The interaction of products (4) with amidine is accompanied by a Smiles rearrangement, which leads to the formation of bicyclic compounds - oxazolopyrimidines (5), which are isosteres of purine bases.
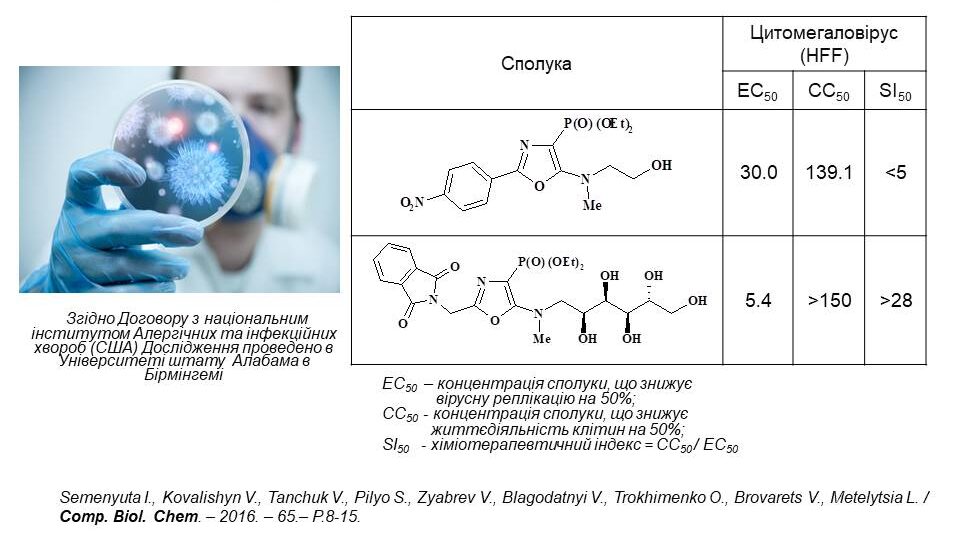
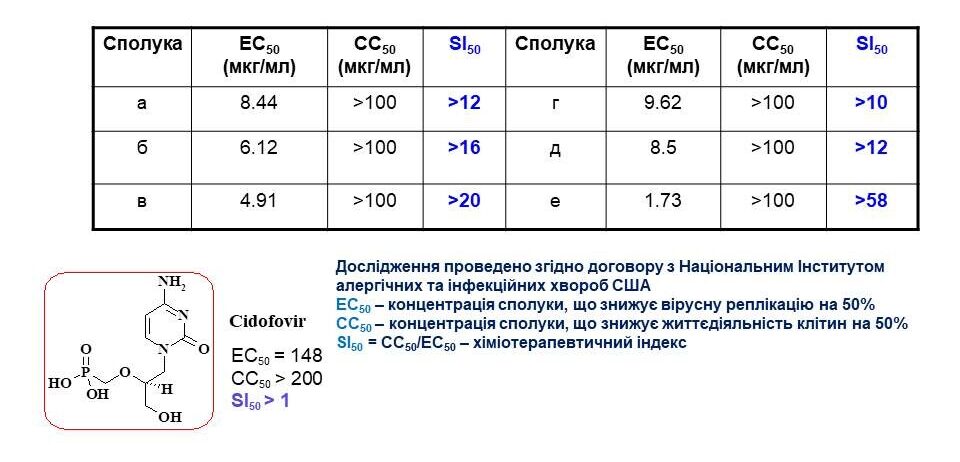
Based on the agreement with the US National Institute of Allergy and Infectious Diseases, the antiviral activity of the synthesised substances was examined. Among them, the leading compounds were found to be aminosulfonyl derivatives of oxazole and thiazole, which proved to be active against human papillomavirus. The slide shows three of the most active representatives of these structures. The structure-activity correlation analysis revealed that 4-aminosulfonyl derivatives of oxazole and thiazole have aryl substituents at positions 2 and 5. In addition, the introduction of a piperidine fragment or a primary amino group into the sulfonyl group is important. For these compounds, the chemotherapeutic index was calculated, which is an order of magnitude higher than the comparison drug Cidofovir. Thus, we have found new classes of substances with antiviral activity. These results encourage further research.
The screening of derivative five- and six-membered nitrogen heterocycles as new effective stimulants of plant growth and development was carried out. Biologically active chemical compounds, derivatives of pyridines, pyrimidines, isoflavonoids, oxazoles, N-substituted sulfamides, which accelerate the growth and development of a number of crops (corn, wheat, soybeans, pumpkin, beans and flax) during ontogeny in vivo and in isolated plant cell cultures in vitro, were selected. According to specific biotests for phytohormonal activity, it was found that chemical compounds in the concentration range of 10-8-10-10M in aqueous solution exhibit high growth and development stimulating activity, which is equal to or exceeds the activity of natural growth regulators and their synthetic analogues auxins and cytokinins: 3-indoleacetic acid (IAA), 1-naphthylacetic acid (NAA) and kinetin. The data obtained indicate the possibility of the use of the selected classes of low molecular weight heterocyclic compounds as new effective plant growth regulators in agriculture and biotechnology.

Group leader: O.V. Golovchenko, Candidate of Chemical Sciences, Senior Research Fellow
Group leader: M.S. Frasynyuk, Doctor of Chemical Sciences, Professor
Group leader: V.A. Tsygankova, Doctor of Biological Sciences, Leading Research Fellow
Group leader: O.D. Kachkovsky, Doctor of Chemical Sciences, Senior Research Fellow
Special attention in the Department is given to rejuvenating the staff of scientific workers. The Department conducts training for highly qualified scientific personnel through full-time postgraduate studies in the specialties 102 "Chemistry" and 091 "Biology and Biochemistry" with the obtained licenses and accredited educational and scientific programs. During the reporting period, the number of PhD candidates, supervised by department employees and who are part of the group ensuring the educational process of the Institute, has increased: in 2018 – 6 postgraduate students, in 2019 – 11 postgraduate students, in 2020 – 15 postgraduate students, in 2021 – 17 postgraduate students, in 2022 – 19 postgraduate students, in 2023 – 25 postgraduate students. After completing their postgraduate studies, most graduates remain to work in the department, replenishing the staff with young specialists. The scientific and pedagogical personnel of Department No. 2 ensure the educational process for training candidates for higher education for the degree of Doctor of Philosophy in the specialties 102 "Chemistry" and 091 "Biology and Biochemistry." The head of the department, Doctor of Chemical Sciences, Corresponding member of the NAS of Ukraine, Volodymyr Brovarets, is the guarantor of the accredited educational and scientific program "Bioorganic Chemistry; Petrochemistry and Hydrocarbons" (specialty 102 "Chemistry"). From 2016 to 2024, 14 students from higher educational institutions completed internships and pre-diploma practices in the department and successfully defended their Master’s theses, of which five Master's students are currently studying in the Institute's postgraduate program. Additionally, six scientific staff and educators from higher education institutions of the Ministry of Education and Science of Ukraine have improved their qualifications in the Department. Currently, there are 33 employees working in the department, including 5 Doctors of Science and 22 Candidates of Science/Doctors of Philosophy. The average age of the researchers in the department is 44 years.
Merzhiyevskiy Danilo Oleksandrovych – SYNTHESIS OF NEW 5-AMINO-1,3-OXAZOLE-4-CARBONITRILES AND STUDY OF THEIR PROPERTIES – 2024.
Brusnakov Mykhailo Yuriyovych – SYNTHESIS AND PROPERTIES OF NEW 4-PHOSPHORYLATED 1,3-OXAZOLES – 2023.
Malets Yegor Serhiyovych – SYNTHESIS AND BIOLOGICAL ACTIVITY OF DERIVATIVES OF 5-, 7-, AND 8-AZACHROMONES – 2023.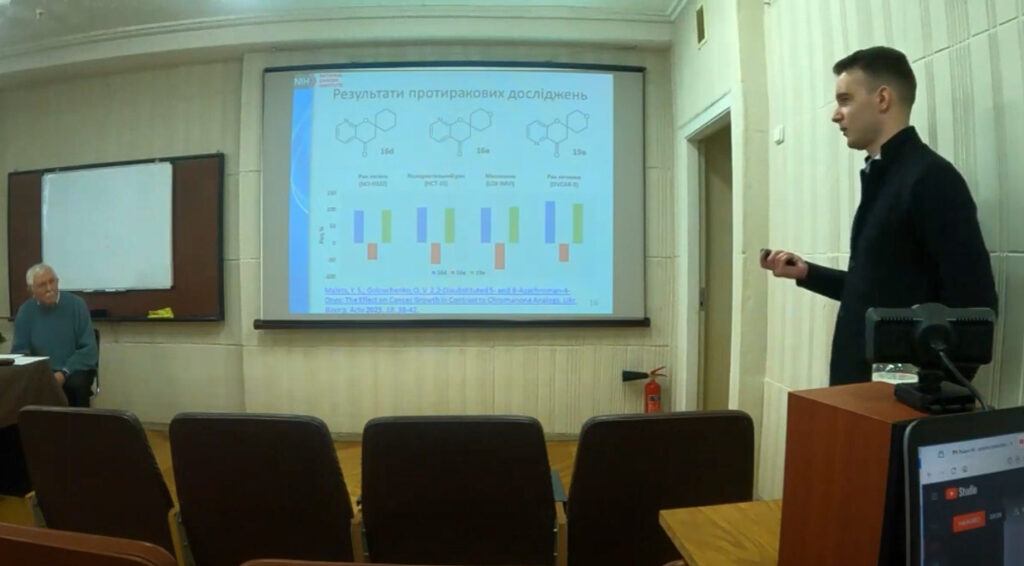
Skalenko Yevhen Oleksandrovych – PHOTOCHEMICAL SYNTHESIS OF DERIVATIVES OF AZABICYCLO[3.2.0]HEPTANES AND AZABICYCLO[4.2.0]OCTANES AND STUDY OF THEIR PROPERTIES – 2023.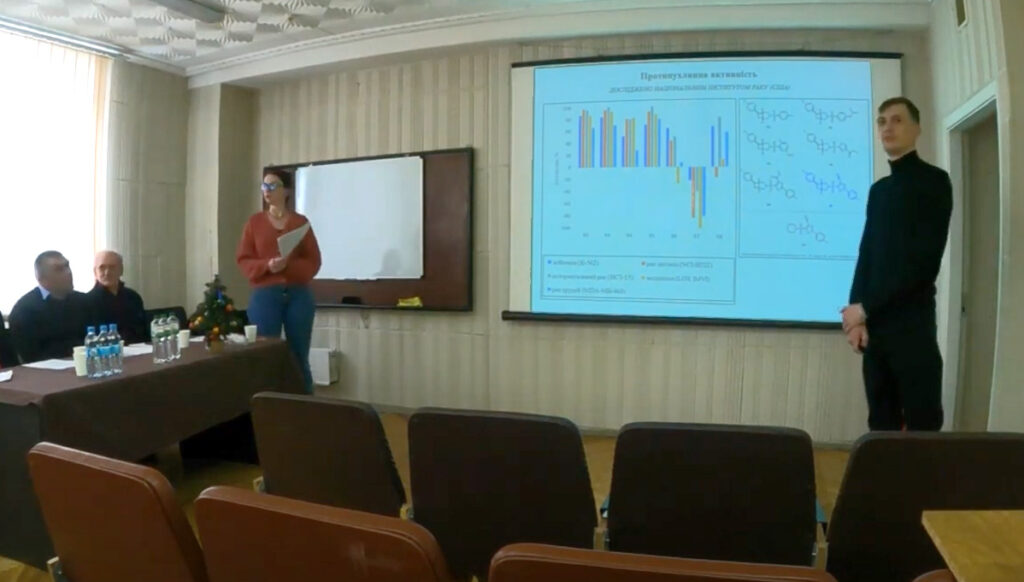
Konovalенко Artem Serhiyovych – 3-ACYL(AZA)ISOCHROMONES IN THE SYNTHESIS OF NEW BIOACTIVE HETERARYL-SUBSTITUTED AND HETEROCONDENSED AZINES – 2023.
Biletska Iryna Mykhailivna – SYNTHESIS AND PROPERTIES OF 2-(3,3,3-TRIFLUOROACETONYL)CHROMONES – 2023.
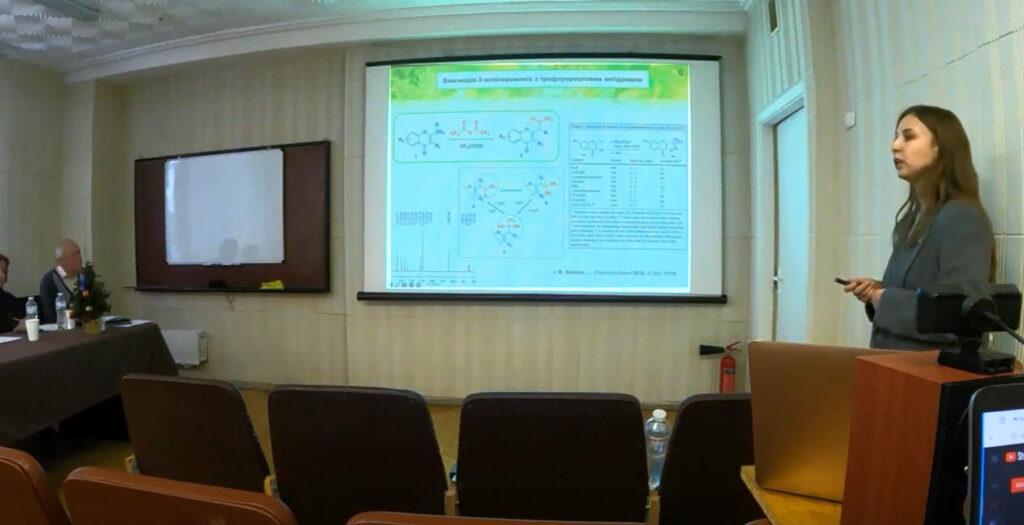
Savych Olena Volodymyrivna – SYNTHESIS AND STUDY OF THE BIOLOGICAL PROPERTIES OF NEW DERIVATIVES OF 3-AMINO-1,2,4-TRIAZOLE AND 5-AMINOTETRAZOLE – 2023.
Velihina Yevheniia Serhiivna – SYNTHESIS AND PROPERTIES OF NEW OXAZOLO[4,5-D]PYRIMIDINES AND PYRAZOLO[1,5-A][1,3,5]TRIAZINES – 2021.
Kornii Yuriy Yevhenovych – SYNTHESIS OF NEW IMINOGIDANTOIN DERIVATIVES WITH ANTIVIRAL AND ANTICANCER ACTIVITY – 2021.
Frasinyuk Mykhailo Serhiyovych – CHEMICAL DESIGN OF BIOLOGICALLY ACTIVE COMPOUNDS BASED ON ISOFLAVONOIDS, AURONES, AND COUMARINS – 2021.
Shtompel Oleksandra Ihorivna – SEARCH FOR PLANT GROWTH REGULATORS AMONG DERIVATIVES OF FIVE- AND SIX-MEMBERED AZAGHETEROCYCLES – 2019.
Solomyanniy Roman Mykolayovych – SYNTHESIS OF BIOACTIVE HETEROCYCLIC COMPOUNDS WITH SULFUR AND PHOSPHORUS-CONTAINING GROUPS BASED ON FUNCTIONALIZED ENAMINES – 2019.

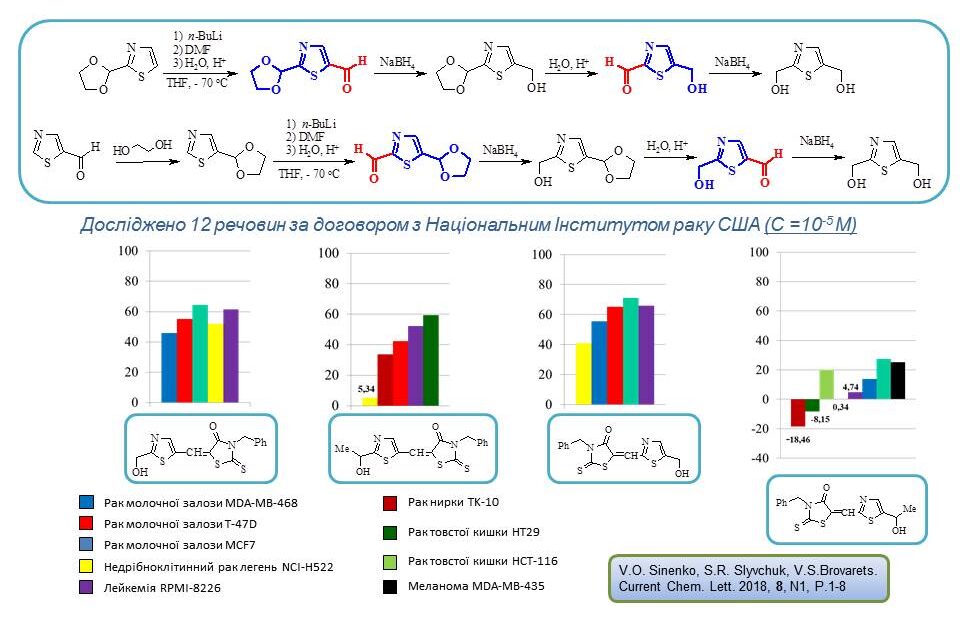
Kachayeva Maria Volodymyrivna – SYNTHESIS AND PROPERTIES OF NEW BIOACTIVE DERIVATIVES OF 1,3-OXAZOLE – 2019.
Syneko Vitaliy Olegovych – OBTAINING NEW BIOLOGICALLY ACTIVE 1,3-THIAZOLES USING LITHIATING AGENTS – 2018.

Popova Antonina Valeriyivna – SYNTHESIS AND PROPERTIES OF NATURAL AURONE ANALOGS – 2018.

Abdurakhmanova Esma Rustemivna – SYNTHESIS AND PROPERTIES OF NEW 4-PHOSPHORYLATED 5-(HYDROXYALKYL)AMINO-1,3-OXAZOLES – 2017.

Korniienko Andrii Mykolayovych – SYNTHESIS AND PROPERTIES OF NEW 1,3-AZOLSUFLONAMIDES – 2015.
Hurenko Artem Olegovych – PROPERTIES OF PYRAZOLO[3,4-d][1,2,3]TRIAZIN-4-ONES AND PRODUCTS OF THEIR TRANSFORMATIONS – 2015.
Lukashuk Olena Ivanivna – SYNTHESIS OF NEW PHOSPHORYLATED PEPTIDOMIMETICS BASED ON DERIVATIVES OF 1,3-OXAZOLE – 2015.
Mruh Halyna Petriivna – SYNTHESIS AND PROPERTIES OF NEW AMINOMETHYL DERIVATIVES OF ISOFLAVONOIDS – 2015.
Chumachenko Svitlana Anatoliyivna – SYNTHESIS AND PROPERTIES OF NEW AZOTIC HETEROCYCLES BASED ON 2-ALKYLOXY-CARBONYLAMINO- AND 2-ALKANOYLAMINO-3,3-DICHLOROACRYLONITRILES – 2013.
Kondratiuk Kostiantyn Mykhailovych – SYNTHESIS AND PROPERTIES OF NEW FUNCTIONALIZED DERIVATIVES OF 1,3-OXAZOLE-4-ILPHOSPHONIC ACID – 2013.
Turov Kostiantyn Volodymyrovych – SYNTHESIS AND PROPERTIES OF NEW DI- AND TRIFUNCTIONALLY SUBSTITUTED 1,3-THIAZOLES – 2012.
Prokopenko Volodymyr Mykhailovych – SYNTHESIS AND PROPERTIES OF 5-N- AND 5-S-SUBSTITUTED DERIVATIVES OF 1,3-OXAZOLE-4-CARBOXYLIC ACID – 2011.
Demydchuk Bohdan Andriyovych – NEW TRANSFORMATIONS OF CHLORINE-CONTAINING ENAMIDES AND 2-AZA-1,3-DIENES INTO DERIVATIVES OF NITROGENOUS HETEROCYCLES – 2009.
Shablykin Oleg Valentynovych – SYNTHESIS OF DERIVATIVES OF 5-AMINO AND 5-HYDRAZINO-1,3-OXAZOLES WITH POTENTIAL BIOREGULATORY PROPERTIES – 2008.
Slyvchuk Serhiy Rostyslavovych – SULFUR-CONTAINING DERIVATIVES OF ACETO- AND ACRYLONITRILES IN THE SYNTHESIS OF BIOREGULATORS OF HETEROCYCLIC NATURE – 2008.
Svyrypa Vitaliy Mykolayovych – NEW TRANSFORMATIONS OF 2-ARYL-4-DICHLOROMETHYLENE-5(4H)-OXAZOLONES AND THEIR ANALOGS – 2007.
Popilnichenko Serhiy Valentynhovych – HETEROCYCLIZATIONS OF β-CHLORO-SUPTITUTED ENAMIDONITRILES WITH N - AND S - NUCLEOPHILES – 2007.
Belyuga Oleksandr Hryhorovych – SYNTHESIS OF BIOACTIVE DERIVATIVES OF NITROGENOUS HETEROCYCLES BASED ON AMIDOPHENACYLATING REAGENTS – 2006.
Holovchenko Oleksandr Volodymyrovych – SYNTHESIS OF NEW BIOREGULATORS OF AZOLE SERIES BASED ON 4,5-DIFUNCTIONALLY SUBSTITUTED OXAZOLES – 2004.
Pilyo Stepan Hryhorovych – SYNTHESIS OF NEW DERIVATIVES OF AZOLES BASED ON 2-ACYLAMINO-3,3-DICHLOROACRYLONITRILES AND THEIR ANALOGS – 2002.
Panchyshyn Svitlana Yaroslavivna – PHOSPHONIUM IONS WITH NITROGEN-CONTAINING GROUPS – PROMISING REAGENTS FOR HETEROCYCLES – 2001.
1. Tang B., Frasinyuk M.S., Chikwana V.M., Mahalingan K.K., Morgan C.A., Segvich D.M., Bondarenko S.P., Mrug G.P., Wyrebek P., Watt D.S., DePaoli-Roach A.A., Roach P.J., Hurley T.D. Discovery and Development of Small-Molecule Inhibitors of Glycogen Synthase. J. Med. Chem. V.63, N 7. 2020. https://doi.org/10.1021/acs.jmedchem.9b01851. 
2. Abdurakhmanova E.R., Brusnakov M.Y., Golovchenko O.V., Pilyo S.G., Velychko N.V., Hariden E.A., Prichard M.N., James S.H., Zhirnov V.V., Brovarets V.S. Synthesis and in vitro anticitomegalovirus activity of 5-hydroxyalkylamino-1,3-oxazoles derivatives. Med. Chem. Res. V.29, N 9. 2020. https://doi.org/10.1007/s00044-020-02593-6
3. Gorgulla C., Boeszoermenyi A., Wang Z., Fischer P.D., Coote P.W., Padmanabha D.K.M, Malets Y.S., Radchenko D.S., Moroz Y.S., Scott D.A., Fackeldey K., Hoffmann M., Iavniuk I.,Wagner G., Arthanari H. An open-source drug discovery platform enables ultra-large virtual screens. Nature V. 580. 2020. https://doi.org/10.1038/s41586-020-2117-z
4. Grygorenko O.O., Moskvina V.S., Hryshchuk O.V., Tymtsunik A.V. Cycloadditions of alkenylboronic derivatives. Synthesis 2020. V.52.https://doi.org/10.1055/s-0040-1707159. 
5. Trush M.M., Kovalishyn V.M., Hodyna D.M., Holovchenko O.V, Chumachenko S.A., Tetko I.V., Brovarets V.S., Metelytsia L.O. In silico and in vitro studies of a number PILs as new antibacterials against MDR clinical isolate Acinetobacter baumannii. Chem. Biol. Drug Des. V.95, 2020. https://doi.org/10.1111/cbdd.13678
6. Velihina Ye., Scattolin T., Bondar D., Pil’o S., Obernikhina N., Kachkovskyi O., Semenyuta I., Caligiuri I., Rizzolio F., Brovarets V., Karpichev Ye., Nolan S.P. Synthesis, in silico and in vitro evaluation of novel oxazolopyrimidines as promising anticancer agents. Helv. Chim. Acta V. 103, N 2. 2020. https://doi.org/10.1002/hlca.202000169
7. Davydenko I.G., Slominskiy Yu.L., Obernikhina N.V., Kachkovsky A.D., Tolmachev A. Infrared Polyene Radical-Cation Derived from 7,8-Dihydrobenzo[c,d]Furo[2,3-f]Indole: Synthesis, Spectra and Nature of Electron Transitions. Chemistry Select V.5. 2020.http://dx.doi.org/10.1002/slct.201904086. 
8. Shcherbakova V., Dibchak D., Snisarenko M., Skalenko Ye., Denisenko A.V., Kuznetsova A.S., Mykhailiuk P.K. Bicyclic piperidines via [2+2] photocycloaddition. J. Org. Chem. V.86, N 3. 2021. https://doi.org/10.1021/acs.joc.0c02355. 
9. Slyvchuk S., Pilyo S., Brovarets V., Kartsev V., Angeli A., Pinteala M., Petrou A., Geronikaki A., Supuran C. Chromene-containing aromatic sulfonamides with carbonic anhydrase inhibitory properties. Int. J. Mol. Sci. V.22, N 10. 2021.https://doi.org/10.3390/ijms22105082
10. Zhirnov V.V., Velihina Ye.S., Mitiukhin O.P., Brovarets V.S. Intrinsic drug potential of oxazolo[5,4-d]pyrimidines and oxazolo[4,5-d]pyrimidines. Chem. Biol. Drug Des. V.98, N 4. 2021.https://doi.org/10.1111/cbdd.13911
11. Angeli A., Kartsev V., Petrou A., Pinteala M., Vydzhak R., Panchishin S., Brovarets V., de Luca V., Capasso C., Geronikaki A., Supuran C. New sulfanilamide derivatives in corporating heterocyclic carboxamide moieties as carbonic anhydrase inhibitors. Pharmaceuticals Vol. 14, N 8. 2021.https://doi.org/10.3390/ph14080828
12. Angeli A., Kartsev V., Petrov A., Pinteala M., Brovarets V., Panchishin S., Vydzhak R., Geronikaki A., Supuran C.T. Carbonic anhydrase inhibition with sulfonamides incorporatiny pyrazole- and pyridazinecarboxamide moieties provides examples of isoform-selective inhibitors. Molecules Vol. 26, N 22. 2021.https://doi.org/10.3390/molecules26227023
13. Ostapiuk Yu.V., Shehedyn M., Barabash O.V., Demydchuk B.A., Batsyts S., Herzberer C., Schmidt A. Bromoarylation of methyl 2-chloroacrylate under Meerwein conditions for the synthesis of substituted 3-hydroxyhiophenes. Synthesis Vol. 53, N 3. 2021.https://doi.org/10.1055/s-0040-1719849. 
14. Piryatinski Yu.P., Verbitsky A.B., Dmytruk A., Malynovskyi M.B., Lutsyk P.M., Rozhin A.G., Kachkovsky O.D., Prostota Ya.O., Kurdyukov V.V. Excited state relaxation in cationic pentamethine cyanines studied by time-resolved spectroscopy. Dyes Pigm. V. 193. 2021.https://doi.org/10.1016/j.dyepig.2021.109539. 
15. Navozenko O., Yashchuk V., Kachkovsky O., Gudeika D., Butkute R., Slominskii Yu., Azovskyi V. Aggregate formation of boron-containing molecules in thermal vacuum deposited films. Materials V. 14, N 19. 2021.https://doi.org/10.3390/ma14195615
16. Bashmakova N.V., Shaydyuk Ye.O., Dmytruk A.M., Swiergosz T., Kachkovsky O.D., Belfield K.D., Bondar M.V., Kasprzyk W. Nature of linear spectral properties and fast electronic relaxations in green fluorescent pyrrolo[3,4-c]pyridine derivative. Int. J. Mol. Sci. V. 22, N 11. 2021.https://doi.org/10.3390/ijms22115592
17. Shaydyuk Ye.O., Bashmakova N.V., Dmytruk A.M., Kachkovsky O.D., Koniev S., Strizhak A.V., Komarov I.V., Belfield K.D., Bondar M.V., Babii O. Nature of fast relaxation processes and spectroscopy of a membrane-active peptide modified with fluorescent amino acid exhibiting excited state intramolecular proton transfer and efficient stimulated emission. ACS Omega. V. 6, N 15. 2021.https://doi.org/10.1021/acsomega.1c00193. 
18. O.O. Grygorenko,V.S. Moskvina,I. Kleban,O.V. Synthesis of saturated and partially saturated heterocyclic boronic derivatives. Tetrahedron. V. 104, N 8. 2022.https://doi.org/10.1016/j.tet.2021.132605. 
19. Metelytsia L.O., Hodyna D.M., Semenyuta I.V., Kovalishyn V.V., Rogalsky S.P., Derevianko K.Yu., Brovarets V.S., Tetko I.V. Theoretical and experimental studies of phosphonium ionic liquids as potential antibacterials of MDR Acinetobacter baumannii. Antibiotics V.11, N 4. 2022. https://doi.org/10.3390/antibiotics11040491
20. Brusnakov M., Golovchenko O., Velihina Ye., Liavynets O., Zhirnov V., Brovarets V. Evaluation of anticancer activity of 1,3-oxazol-4-ylphosphonium salts in vitro. Chem. Med. Chem. V.17, N 20. 2022.https://doi.org/10.1002/cmdc.202200319. 
21. Frasinyuk M., Chhabria D., Kartsev V., Dilip H., Sirakanyan S.N., Kirubakaran S., Petrou A., Geronikaki A., Spinelli D. Benzothiazole and chromone derivatives as potential ATR kinase inhibitors and anticancer agents. Molecules V.27, N 14. 2022.https://doi.org/10.3390/molecules27144637
22. Waszkowska K., Krupka A., Smokal V., Kharchenko O., Migalska-Zalas A., Frasinyuk M., Wielgosz R., Andrushchak A., Sahraoui B. Correlation between nonlinear optical effects and structural features of aurone-based methacrylic polymeric thin films. Materials V.15, N 17. 2022.https://doi.org/10.3390/ma15176076
23. Moskvina V.S., Kleban I., Hryshchyk O.V., Grygorenko O.O. Synthesis of saturated and partially saturated heterocyclic boronic derivatives. Tetrahedron V.104. 2022.https://doi.org/10.1016/j.tet.2021.132605. 
24. Kornii Yu., Shablykin O., Tarasiuk T., Stepaniuk O., Matvienko V., Aloshyn D., Zahorodniuk N., Sadkova I.V., Mykhailiuk P.K. Fluorinatedaliphatic diazirines: preparation, characterization, and model photolabeling studies. J. Org. Chem. V.88, N 1. 2022.https://doi.org/10.1021/acs.joc.2c02262. 
25. Zhirnov V., Shablykin O., Chumachenko S., Kornii Yu., Keith K. A., Harden E. M., Hartline C. B., James S. H., Kobzar O., Kovalishyn V., Vovk A., Brovarets V. In vitro activity of novel 4-iminohydantoin sulfamide derivatives against human cytomegalovirus. Chem. Papers. V. 78. 2023.https://doi.org/10.1007/s11696-023-03038-1
26. Nizhenkovska I. V., Matskevych K. V., Golovchenko O. I., Golovchenko O. V., Kustovska A. D., Van M. New prospective phosphodiesterase inhibitors: phosphorylated oxazole derivatives in treatment of hypertension. Adv. Pharm. Bull. V.13, N 2. 2023.https://doi.org/10.34172/apb.2023.044
27. Metelytsia L. O., Hodyna D. M., Semenyuta I. V., Kovalishyn V. V., Rogalsky S. P., Derevianko K. Yu., Brovarets V. S., Tetko I. V. Theoretical and experimental studies of phosphonium ionic liquids as potential antibacterials of MDR. Antibiotics. V.11, N 4. 2022.https://doi.org/10.3390/antibiotics11040491
28. Velihina Ye., Gesese R., Zhirnov V., Kobzar O., Bui B., Pilyo S., Vovk A., Shen H., Brovarets V. Design, synthesis and evaluation of the anti-breast cancer activity of 1,3-oxazolo[4,5-d]pyrimidine and 1,3-oxazolo[5,4-d]pyrimidine derivatives. RCS Med. Chem. V.14, N 4. P. 2023.https://doi.org/10.1039/D2MD00377E.
29. Bondar D., Bragina O., Lee Ji Y., Semenyuta I., Jӓrving I., Brovarets V., Wipf P., Bahar I., Karpichev Y. Hydroxamic Acids as PARP-1 Inhibitors: Molecular Design and Anticancer Activity of Novel Phenanthridinones. Helv. Chim. Acta. V.106, N 10. 2023.https://doi.org/10.1002/hlca.202300133
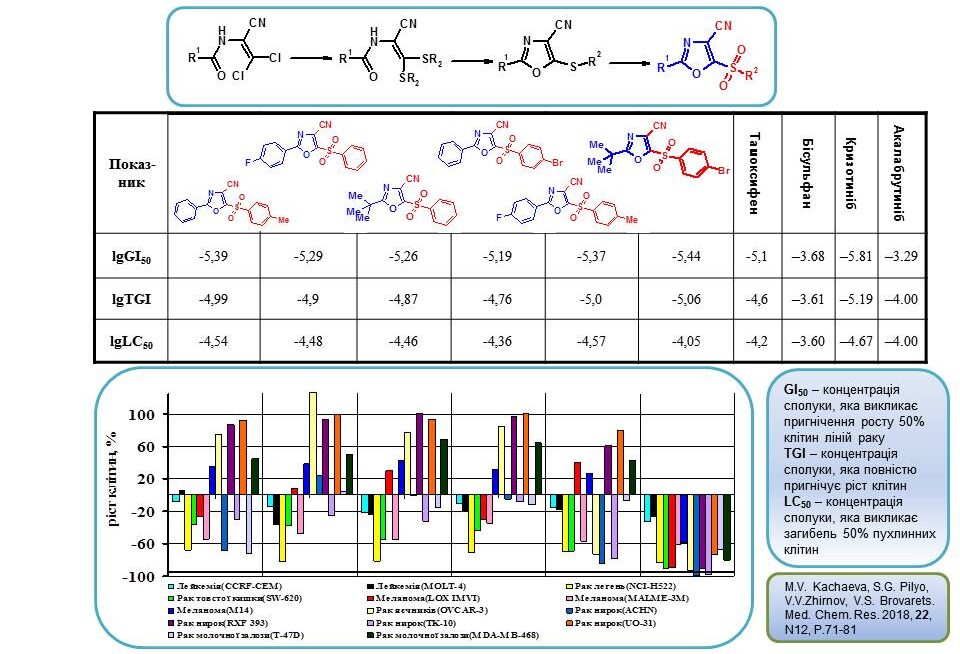
30. Zyabrev V., Pilyo S., Demydchuk B., Kachaeva M., Semenyuta I., Zhirnov V., Velihina Ye., Brovarets V. Synthesis, characterization and in vitro anticancer evaluation of 5-sulfinyl(sulfonyl)-4-arylsulfonyl substituted 1,3-thiazoles. Chem. Med. Chem. V.18, N 14. 2023.https://doi.org/10.1002/cmdc.202300161. 
31. Konovalenko A.S., Shablykin O.V., Shablykina O.V., Moskvina V.S., Shishkina S.V., Kozytskyi A.V., Brovarets V.S. Distinctive features of 3-acetyl- and 3-benzoylisocoumarins’ interaction with active primery amines. Chem. Select. V.8, N 37. 2023.https://doi.org/10.1002/slct.202301380. 
32. Shaydyuk Ye.O., Bashmacova N.V., Klishevich G.V., Dmytruk A.M., Kachkovsky O.D., Kuziv Ya.B., Dubey I.Ya., Befield K.D., Bondar M.V. Nature of linear spectra; properties and fast relaxations in the excited states and two-photon absorption efficiency of 3-thiazolyl and 3-phenylthiazolyl coumarin derivatives. ACS Omega. V.18, N 12. 2023.https://doi.org/10.1021/acsomega.3c00654. 
33. Nallaparaju J.V., Nikonovich T., Jarg T., Merzhyievskyi D., Aav R., Kananovich D.G. Mechanochemistry – amended barbier reaction as an expedient alternative to Grignar synthesis. Angew. Chem. Int. Edd. V.62, N. 39. 2023.https://doi.org/10.1002/anie.202305775. 
34. Hodyna D., Kovalishyn V., Romanenko Ya., Semenyuta I., Blagodatny V., Kachaeva M., Brazhko O., Metelytsia L. Quinoline hydrazine derivatives as new antibacterials against multidrug resistant strains. Chem. Biodiversity V.20. 2023.https://doi.org/10.1002/cbdv.202300839. 
35. Dibchak D., Snisarenko M., Mishuk A., Shablykin O., Bortnichuk L., Klymenko-Ulianov O., Kheylik Yu., Sadkova I., Rzepa H.S., Mykhailiuk P.K. General synthesis of 3‐azabicyclo [3.1.1]heptanes and evaluation of their properties as saturated isosteres. Angewandte Chemie. V.135, N 39. 2023. https://doi.org/10.1002/ange.202304246. 
36. Kirichok A., Tkachuk H., Kozyriev Ye., Shablykin O., Datsenko O., Granat D., Yegorova T., Bas Yu., Semirenko V., Pishel I., Kubyshkin V., Lesyk D., Klymenko-Ulianov O., Mykhailiuk P.K. 1‐Azaspiro[3.3]heptane as a bioisostere of piperidine. Angewandte Chemie. e202311583. V.135, N 51. 2023.https://doi.org/10.1002/ange.202311583. 
37. Chernykh A.V., Kudryk O.V., Olifir O.S., Dobrydnev A.V., Rusanov E., Moskvina V.S., Volochnyuk D.M., Grygorenko O.O. Expanding the chemical space of 1,2-difunctionalized cyclobutanes. J. Org. Chem. V.88, N5. 2023.https://doi.org/10.1021/acs.joc.2c02892. 
38. Chen X., Lv X., Gao L., Liu J., Wang W., Guo L., Frasinyuk M. S., Zhang W., Watt D. S., Liu C., Liu X. Chalcone derivative CX258 suppresses colorectal cancer via inhibiting the TOP2A/Wnt/β-Catenin signaling. Cells. V.12, N 7. 2023. https://doi.org/10.3390/cells12071066.
39. Mrug G., Hodyna D., Metelytsia L., Kovalishyn V., Trokhimenko O., Bondarenko S., Kondratyuk K., Kozitskiy A., Frasinyuk M. Structure-activity relationship prediction-based synthesis and cytotoxicity evaluation against the HEp-2 laryngeal carcinoma cell of isoflavone–cytisine mannich bases. Chem. Biodivers. V.20, N 8. 2023.https://doi.org/10.1002/cbdv.202300560. 
40. Myshko A., Mrug G., Kondratyuk K., Demydchuk B., Bondarenko S., Frasinyuk M. An expedient synthesis of functionalized pyrazole-based aurone analogs. Chemistry Select. V.8, N 20. 2023.https://doi.org/10.1002/slct.202300257. 
41. Levterov V., Panasyuk Ya., Sahun K., Stashkevich O., Badlo V., Shablykin O., Sadkova I., Bortnichuk L., Klymenko-Ulianov O., Holota Yu., Lachmann L., Borysko P., Horbatok K., Bodenchuk I., Bas Yu., Dudenko D., Mykhailiuk P.K. 2-Oxabicyclo [2.2.2]octane as a new bioisostere of the phenyl ring. Nature Commun. V.14, N 1. 2023. https://doi.org/10.1038/s41467-023-41298-3
42. Goulden T., Bodachivskyi Iu., Padula M.P., Williams D.B.G. Concentrated ionic liquids for proteomics: Caveat emptor! Concentrated ionic liquids for proteomics: Caveat emptor! International Journal of Biological Macromolecules. V.253, N 7. 2023. https://doi.org/10.1016/j.ijbiomac.2023.127438. 
43. Kornii Yu., Shablykin O., Tarasiuk T., Stepaniuk O., Matvienko V., Aloshyn D., Zahorodniuk N., Sadkova I. V., Mykhailiuk P. K. Fluorinatedaliphatic diazirines: preparation, characterization, and model photolabeling studies. J. Org. Chem. V.88, N 1. 2023.https://doi.org/10.1021/acs.joc.2c02262. 
44. Bondar M.V., Faryadras S., Munera N., Chang H.-T., Uddin M., Beldield K.D., Kachkovsky O.D., Van Stryland E.W., Hagan D.J. New two-photon absorbing Squaraine derivative with efficient near-infrared fluorescence, superluminescence, and high photostability. J. Phys. Chem. B. V.126, N 21. 2022.https://doi.org/10.1021/acs.jpcb.2c01288. 
45. Denisenko A., Garbuz P., Makovetska Ye., Shablykin O., Lesyk D., Al-Maali G., Korzh R., Sadkova I., Mykhailiuk P. 1,2-Disubstituted bicyclo[2.1.1]hexanes as bioisosteres of the ortho-substituted benzene. Chem. Sci. V. 14. 2023. https://doi.org/10.1039/D3SC05121H.
46. Konovalenko A., Shablykin O., Shablykina O., Kozytskyi A., Brovares V. Convenient and versatile method of 8‑amino-6-(2-R-thiazol-4-yl)1,7-naphthyridines synthesis. Curr. Chem. Lett. 2024. V.13, N 1. P. 163-172. http://dx.doi.org/10.5267/j.ccl.2023.7.004.
47. Prysiazhniuk K., Datsenko O., Polishchuk O., Shulha S., Shablykin O., Nikandrova L., Horbatok K., Bodenchuk I., Borysko P., Shepilov D., Pishel I., Kubyshkin V., Mykhailiuk P. Spiro[3.3]heptane as a saturated benzene bioisostere. Angewandte Chemie. Int. Ed. Vol. 63, N 9. 2024.https://doi.org/10.1002/anie.202316557. 
48. Severin O., Kachaeva M., Pilyo S., Zhirnov V., Hodyna D., Bahrieieva O., Brovarets V. Synthesis, characterization of novel N-(4-cyano-1,3-oxazol-5-yl)sulfonamide derivatives and in vitro screening of their activity against NCI-60 cancer cell lines. ChemMedChem. V. 19, N5.2024.https://doi.org/10.1002/cmdc.202300527. 
49. Zyabrev V., Demydchuk B., Pilyo S., Zhirnov V., Liavynets O., Brovarets V. Synthesis, characterization, and in vitro anticancer evaluation of 2,4-disulfonyl-substituted 5-aminothiazoles. Curr Chem Lett. Vol. 13, N 3. 2024.https://doi.org/10.5267/j.ccl.2024.2.003
50. Nallaparaju J.V., Satsi R., Merzhyievskyi D., Jarg T., Aav R., Kananovich D.G. Mechanochemical birch reduction with low reactive alkaline earth metals. Angew Chem Intern Ed. V. 63, N 20. 2024.https://doi.org/10.1002/anie.202319449. 
51. Semenyuta I., Golovchenko O., Bagreeva O., Vydzhak R., Zhirnov V., Brovarets V. Synthesis, characterization, in vitro anticancer evaluation, ADMET properties, and molecular docking of novel 5-sulfonyl substituted (thiazol-4-yl)posphonium salts. ChemMedChem. V.19, N 18. 2024.https://doi.org/10.1002/cmdc.202400205. 
52. Bondarchuk T., Vaskiv D., Zhuravel E., Shyshlyk O., Hrynyshyn Ye., Nedialko O., Pokholenko O., Pohribna A., Kuchuk O., Brovarets V., Zozulya S. Synthetic amine lincers for efficient sortagging. Bioconjugate Chem. Vol. 35, N 8. 2024. https://doi.org/10.1021/acs.bioconjchem.4c00143. 
53. Sinenko V.O., Los O.V., Potikha L.M., Brovarets V.S. Functionalized 1,3-thiazoles by combined halogen dance. Curr Chem Lett. Vol. 13, N 4. 2024.https://doi.org/10.5267/j.ccl.2024.5.001.
54. Hodyna D, Klipkov A., Kachaeva M., Shulga Y., Gerus I., Metelytsia L., Kovalishyn V. In silico design and in vitro assessment of bicyclic trifluorometylated pyrroles as new antibacterial and antifungal agents. Chemistry and Biodiversity. Vol. 21, N8. 2024.https://doi.org/10.1002/cbdv.202400638. 
55. Levterov V.V., Panasiuk Ya., Shablykin O., Stashkevych O., Sahun K., Rassokhin A., Sadkova I., Lesyk D., Anisiforova A., Holota Yu., Borysko P., Bodenchuk I., Voloshchuk N.M., Mykhailiuk P.K. 2-Oxabicyclo[2.1.1]hexanes: synthesis, properties, and validation as bioisosteres of ortho- and meta-benzenes. Angew. Chem. Int. Ed. Vol. 63, N 19. 2024.https://doi.org/10.1002/anie.202319831. 
56. Prysiazhniuk K., Datsenko O.P., Polishchuk O., Shulha S., Shablykin O., Nikandrova Ye., Horbatok K., Bodenchuk I., Borysko P., Shepilov D., Pishel I., Kubyshkin V., Mykhailiuk P.K. Spiro[3.3]heptane as a saturated benzene bioisostere. Angew. Chem. Int. Ed. V.63, N 9. 2024.https://doi.org/10.1002/anie.202316557. 
57. Kobrina L., Boiko V., Shtompel V., Hudzenko N., Rogalsky S., Frasinyuk M., Kozitskiy A., Riabov S. Inclusion complex of ionic liquid 1-dodecylpyridinium tetrafluoroborate with sulfobutyl ether-β-cyclodextrin: Preparation and characterization. J. Mol. Struct. V.1309. 2024.http://doi.org/10.1016/j.molstruc.2024.138137. 
58. Govor E. V., Naumchyk V., Nestorak I., Radchenko D.S., Dudenko D., Moroz Yu.S., Kachkovsky O.D., Grygorenko O.O. Generation of multimillion chemical space based on the parallel Groebke–Blackburn–Bienaymé reaction. Beilstein J. Org. Chem. V. 20. 2024.https://doi.org/10.3762/bjoc.20.143.
59. Chernykh A.V., Kudryk O.V., Olifir O.S., Dobrydnev A.V., Rusanov E., Moskvina V.S., Volochnyuk D.M., Grygorenko O.O. Expanding the chemical space of 1,2-difunctionalized cyclobutanes. J. Org. Chem.. Vol. 88, N 5. 2023.https://doi.org/10.1021/acs.joc.2c02892. 
60. Gryniukova A., Borysko P., Myziuk I., Alieksieieva D., Hodyna D., Semenyuta I., Kovalishyn V., Metelytsia L., Rogalsky S., Tcherniuk S. Anticancer activity features of imidazole-based ionic liquids and lysosomotropic detergents: in silico and in vitro studies. Molecular Diversity V. 28. 2024.https://doi.org/10.1007/s11030-023-10779-4.
61. Bondarchuk T., Vaskiv D., Zhuravel E., Shyshlyk O., Hrynyshyn Ye., Nedialko O., Pokholenko O., Pohribna A., Kuchuk O., Brovarets V., Zozulya S. Synthetic amine linkers for efficient sortagging. Bioconj. Chem. V.35, N8. 2024. https://doi.org/10.1021/acs.bioconjchem.4c00143. 
62. Demchenko S., Yarmoluk S., Sukhovieiev V., Golovchenko O., Sukhovieiev O., Demchenko A. Syntheses and evaluation of novel 3-hydroxy-1,3-diaryl-2, 3,5,6,7,8-hexahydroimidazo[1,2-a]-pyridine-1-ium bromides as potential anticancer agents. Pharmacia Vol.71. 2024.https://doi.org/10.3897/pharmacia.71.e135992
63. Tsygankova V.A., Andrusevich Ya.V., Vasylenko N.M., Kopich V.M., Popilnichenko S.V., Pilyo S.G., Brovarets V.S. Auxin-like and cytokinin-like effects of new synthetic pyrimidine derivatives on the growth and photosynthesis of wheat. J Plant Sci Phytopathol. V.8, N1, 2024. https://dx.doi.org/10.29328/journal.jpsp.1001126
64. Blyuss K. B., Fatehi F., Tsygankova V. A., Biliavska L. O., Iutynska G. O., Yemets A. I., Blume Y. B. RNAi-Based biocontrol of wheat nematodes using natural poly-component biostimulants. Front. Plant Sci. V.10. 2019. https://doi.org/10.3389/fpls.2019.00483.
65. Krupodorova T., Barshteyn V., Tsygankova V., Sevindik M., Blume Y. Strain-specific features of Pleurotus ostreatus growth in vitro and some of its biological activities. BMC Biotechnol. V.24, N9. 2024. https://doi.org/10.1186/s12896-024-00834-9
66. Pidlisnyuk V., Mamirova A., Newton R.A., Stefanovska T., Zhukov O., Tsygankova V., Shapoval P. The Role of Plant Growth Regulators in Miscanthus × giganteus Growth on Trace Elements-Contaminated Soils Agronomy Vol.12, N12. 2022. https://doi.org/10.3390/agronomy12122999
The search for bioactive drugs among synthesised azaheterocycles was carried out by employees of other biological departments of our Institute, as well as specialists from the Institute of Molecular Biology and Genetics of the National Academy of Sciences of Ukraine, the Institute of Botany of the National Academy of Sciences of Ukraine, Zaporizhia Medical University, the University of Kentucky (USA), the National Institutes of Health (USA), the National Institute for Cancer Research (USA), and the Open Innovative Platform BASF (Germany).

According to the Agreement signed in 2018 between the V.P. Kukhar IBOPC of the NAS of Ukraine, BASF is engaged in the research, development, production and marketing of agrochemical products and is interested in further developing compounds suitable for use in agrochemical products. BASF has created an open innovation agro-industrial platform to check whether compounds provided by third parties can be used as a basis for the development of agrochemical products and to seek cooperation in the field of agreements ('Open Innovation Platform Agro – OIP AGRO').

The V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences of Ukraine, in accordance with the NIAID Nonclinical Testing Agreement ('NCEA'), 2018, participated in programs supported by the Division of Microbiology and Infectious Diseases (DMID), a division of the National Institute of Allergy and Infectious Diseases ('NIAID'), an institution of the National Institutes of Health, which is part of the Department of Health and Human Services (HSS), an agency of the United States Government. Approximately 400 newly synthesised substances were tested for possible activity against infectious organisms and viruses.

In 2013, the V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences of Ukraine signed an Agreement with the US National Cancer Institute, and during this period, more than 1,500 substances were screened for possible use in cancer chemotherapy.

In 2025, an Agreement was signed with the Institute of Medical Biology of the Polish Academy of Sciences on the joint use of rights to the invention project ‘Naphthalimide derivative, method of its production and application.’
Viktoria Tsygankova, Leading Research Fellow, Doctor of Biological Sciences, Senior Research Fellow
https://orcid.org/0000-0002-8036-6488
http://irbis-nbuv.gov.ua/ASUA/0008330
https://scholar.google.com.ua/citations?user=hDZtSNwAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=55816912700
Viktor Zhirnov, Leading Research Fellow, Doctor of Medical Sciences, Senior Research Fellow
https://orcid.org/0000-0001-7453-7124
http://irbis-nbuv.gov.ua/ASUA/1264160
https://scholar.google.com.ua/citations?user=CGX7c9IAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=8430489400
Mykhaylo Frasyniuk, Professor, Doctor of Chemical Sciences, Senior Research Fellow
https://orcid.org/0000-0003-3133-601X
http://irbis-nbuv.gov.ua/ASUA/0222999
https://scholar.google.com.ua/citations?user=jZdF2pgAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=8920555400
Volodymyr Zyabrev, Senior Research Fellow, Candidate of Chemical Sciences, Senior Research Fellow
https://orcid.org/0000-0002-1383-2100
http://irbis-nbuv.gov.ua/ASUA/1260158
https://scholar.google.com.ua/citations?user=N8u7TOwAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=6701325265
Stepan Pilyo, Senior Research Fellow, Candidate of Chemical Sciences, Senior Researcher
https://orcid.org/0000-0002-7089-1393
http://irbis-nbuv.gov.ua/ASUA/0028699
https://scholar.google.com.ua/citations?user=No5xZzgAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=6602526195
Oleh Mitiukhin, Senior Research Fellow, Candidate of Chemical Sciences
https://orcid.org/0000-0001-7839-2428
http://irbis-nbuv.gov.ua/ASUA/1261105
https://scholar.google.com.ua/citations?user=RNV_Rp0AAAAJ
https://www.scopus.com/authid/detail.uri?authorId=7801580069
Oleksandr Golovchenko, Senior Research Fellow, Candidate of Chemical Sciences, Senior Researcher
https://orcid.org/0000-0001-7756-6019
http://irbis-nbuv.gov.ua/ASUA/0031480
https://scholar.google.com.ua/citations?user=cUPS7hMAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=35096146700
Oleh Shablykin, Senior Research Fellow, Candidate of Chemical Sciences, Senior Researcher
https://orcid.org/0000-0001-6810-9860
http://irbis-nbuv.gov.ua/ASUA/0021461
https://scholar.google.com.ua/citations?user=35FD-MkAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=15760599300
Olexiy Kachkovsky, Senior Research Fellow, Doctor of Chemical Sciences
https://orcid.org/0000-0003-3711-5154
http://irbis-nbuv.gov.ua/ASUA/1258030
https://scholar.google.com.ua/citations?user=PukBwH0AAAAJ
https://www.scopus.com/authid/detail.uri?authorId=6603799170
Yaroslav Andrusevych, Senior Research Fellow, Candidate of Biological Sciences
https://orcid.org/0009-0002-0330-0925
http://irbis-nbuv.gov.ua/ASUA/0060718
https://scholar.google.com.ua/citations?user=Hy73XsQAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=55560412600
Roman Vydzhak, Senior Research Fellow, Candidate of Chemical Sciences
https://orcid.org/0000-0001-7942-169X
http://irbis-nbuv.gov.ua/ASUA/1483650
https://scholar.google.com.ua/citations?user=-LasqVIAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=6603661011
Iurii Bodachivskyi, Senior Research Fellow, Candidate of Chemical Sciences
https://orcid.org/0000-0001-5803-4674
http://irbis-nbuv.gov.ua/ASUA/1466162
https://www.scopus.com/authid/detail.uri?authorId=57195505410
Ivan Semenyuta, Senior Research Fellow, Candidate of Biological Sciences, Senior Researcher
https://orcid.org/0000-0001-8464-3692
http://irbis-nbuv.gov.ua/ASUA/0019299
https://scholar.google.com.ua/citations?user=ognQC5sAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=6505779360
Sergii Slyvchuk, Research Fellow, Candidate of Chemical Sciences
https://orcid.org/0009-0004-6936-4870
http://irbis-nbuv.gov.ua/ASUA/0033104
https://scholar.google.com.ua/citations?user=_pm8xy0AAAAJ
https://www.scopus.com/authid/detail.uri?authorId=55220935000
Kostyantyn Kondratyuk, Research Fellow, Candidate of Chemical Sciences
https://orcid.org/0000-0001-9191-9933
http://irbis-nbuv.gov.ua/ASUA/0060763
https://scholar.google.com.ua/citations?user=fs3WhRIAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=8891058700
Svitlana Chumachenko, Research Fellow, Candidate of Chemical Sciences
https://orcid.org/0000-0001-7020-8622
http://irbis-nbuv.gov.ua/ASUA/0061107
https://scholar.google.com.ua/citations?user=UbH8WPUAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=36674573300
Bohdan Demydchuk, Research Fellow, Candidate of Chemical Sciences
https://orcid.org/0000-0002-9125-3796
http://irbis-nbuv.gov.ua/ASUA/0022839
https://scholar.google.com.ua/citations?user=cFFiXsMAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=14053785700
Oleksandr Kozachenko, Research Fellow, Candidate of Chemical Sciences
https://orcid.org/0009-0009-1212-7424
http://irbis-nbuv.gov.ua/ASUA/0046668
https://scholar.google.com.ua/citations?user=ODS7G1kAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=57026495200
Svitlana Panchishin, Research Fellow, Candidate of Chemical Sciences
https://orcid.org/0000-0001-5763-8476
http://irbis-nbuv.gov.ua/ASUA/1261181
https://scholar.google.com.ua/citations?user=g-MWkFkAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=6602733421
Maryna Kachaeva, Research Fellow, Candidate of Chemical Sciences
https://orcid.org/0000-0003-1517-4807
http://irbis-nbuv.gov.ua/ASUA/1267374
https://scholar.google.com.ua/citations?user=FgSQfhsAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=57194614164
https://www.webofscience.com/wos/author/record/AAZ-5596-2021
https://www.webofscience.com/wos/author/record/2380947
Vitaliy Sinenko, Research Fellow, Candidate of Chemical Sciences
https://orcid.org/0009-0000-5677-3692
http://irbis-nbuv.gov.ua/ASUA/1268855
https://scholar.google.com.ua/citations?user=POQ8NAMAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=56878916900
Roman Solomiannyi, Junior Research Fellow, Candidate of Chemical Sciences
https://orcid.org/0009-0006-2275-6759
http://irbis-nbuv.gov.ua/ASUA/1268977
https://scholar.google.com.ua/citations?user=qsNbXbsAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=24823376300
Artem Konovalenko, Junior Research Fellow, PhD
https://orcid.org/0000-0003-3538-2814
Mykhailo Brusnakov, Junior Research Fellow, PhD
https://orcid.org/0000-0002-8814-7984
http://irbis-nbuv.gov.ua/ASUA/1488080
https://scholar.google.com.ua/citations?user=fDbGQqkAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=57214441083
https://www.webofscience.com/wos/author/record/ENX-0957-2022
https://www.webofscience.com/wos/author/record/19771351
Viktor Kopich, Junior Research Fellow, Candidate of Biological Sciences
https://orcid.org/0009-0009-2240-996X
Yehor Malets, Junior Research Fellow, PhD
https://orcid.org/0000-0002-3029-3065
http://irbis-nbuv.gov.ua/ASUA/1490134
https://scholar.google.com.ua/citations?user=CWtg7YkAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=57212171613
Yevheniia Velihina, Junior Research Fellow, PhD
https://orcid.org/0000-0003-1792-9919
http://irbis-nbuv.gov.ua/ASUA/1484366
https://scholar.google.com.ua/citations?user=uS1TMg0AAAAJ
https://www.scopus.com/authid/detail.uri?authorId=57209311302
Nataliya Lyutenko, Junior Research Fellow, PhD
https://orcid.org/0000-0003-3538-2814
http://irbis-nbuv.gov.ua/ASUA/0031017
https://scholar.google.com.ua/citations?user=mhr5qc8AAAAJ
https://www.scopus.com/authid/detail.uri?authorId=6507103527
Yurii Kornii, Junior Research Fellow, Candidate of Chemical Sciences
https://orcid.org/0000-0003-4266-8773
Oksana Bahrieieva, 1 Category Engineer
https://orcid.org/0009-0008-6108-3045
https://www.scopus.com/authid/detail.uri?authorId=58557321700
Oleksandr Severin, Junior Research Fellow, PhD
https://orcid.org/0000-0003-3943-5063
http://irbis-nbuv.gov.ua/ASUA/1491637
https://scholar.google.com.ua/citations?user=S4F7XIwAAAAJ
https://www.scopus.com/authid/detail.uri?authorId=57486019500
https://www.webofscience.com/wos/author/record/DQJ-8451-2022
https://www.webofscience.com/wos/author/record/14078856
Andrii Myshko, Junior Research Fellow, PhD
https://orcid.org/0009-0007-3673-120X
Yehor Herasymov, 1 Category Engineer
https://orcid.org/0009-0003-2062-2480
Oleksandr Los, 1 Category Engineer
In order to implement the results of scientific activities, the Department's employees cooperate with institutions and organisations of the production sector within the framework of economic agreements.
In particular, work is being carried out with LLC 'SPE "Ukrorgsyntez' related to the development of preparative syntheses of bioactive compounds of heterocyclic nature: azoles, azines and their condensed derivatives.
Scientists of the group of biological screening of synthetic compounds of the Department, headed by the leading researcher, Doctor of Biological Sciences, V.A. Tsygankova, conduct joint scientific and technical cooperation with the Agricultural company 'Peremoga' (village Malopolovetske, Fastiv district, Kyiv region). Joint tests of plant growth regulators synthesised in the Department on the yield of spring durum wheat of the Demira variety are being conducted in the fields of the Agricultural company 'Peremoga'. Based on the results of field tests, a methodology for pre-sowing treatment of wheat seeds with plant growth regulators has been developed, and an assessment of the economic efficiency of the developed plant growth regulators has been carried out. In accordance with the Act on Field Testing of Plant Growth Regulators, the Agricultural company Peremoha recommends the introduction of growth regulators developed in the Department into agricultural practice, which will contribute to solving the problem of the food crisis under martial law, providing the population with food and preserving the environment, and will also allow saving state funds on the purchase of expensive foreign-made plant growth regulators.

Some results of the implementation of the researchers of the Department No. 2 in the national economy

















![12-16 листопада 2018 року делегація Інституту біоорганічної хімії та нафтохімії ім. В.П. Кухаря НАН України брала участь у 8-й Міжнародній конференції “Хімія азотовмісних гетероциклів” присвячену пам’яті професора Валерія Орлова (8th International Conference “CHEMISTRY OF NITROGEN CONTAINING HETEROCYCLES” in memoriam of Prof. Valeriy Orlov), м. Харків. Було представлено три усні доповіді: Dr. Esma Abdurakhmanova “New phosphonopeptidomimetics: synthesis and properties” Dr. Oleksandr Kozachenko “Syntheses of new azolopyrimidines on the basis of 2 acylamino-3,3-dichloroacrylonitriles” Dr. Maryna Kachaeva “Anticancer activity of new 1,3-oxazole derivatives: in silico and in vitro” Аспіранка відділу №2 Євгенія Велігіна була нагороджена дипломом за найкращу стендову доповідь “Synthesis, characterization, and in vitro anticancer evaluation of 7-piperazin-substituted [1,3]oxazolo[4,5-d]pyrimidines“.](https://bpci.kiev.ua/wp-content/uploads/2025/04/image01-1024x495.jpg)
![12-16 листопада 2018 року делегація Інституту біоорганічної хімії та нафтохімії ім. В.П. Кухаря НАН України брала участь у 8-й Міжнародній конференції “Хімія азотовмісних гетероциклів” присвячену пам’яті професора Валерія Орлова (8th International Conference “CHEMISTRY OF NITROGEN CONTAINING HETEROCYCLES” in memoriam of Prof. Valeriy Orlov), м. Харків. Було представлено три усні доповіді: Dr. Esma Abdurakhmanova “New phosphonopeptidomimetics: synthesis and properties” Dr. Oleksandr Kozachenko “Syntheses of new azolopyrimidines on the basis of 2 acylamino-3,3-dichloroacrylonitriles” Dr. Maryna Kachaeva “Anticancer activity of new 1,3-oxazole derivatives: in silico and in vitro” Аспіранка відділу №2 Євгенія Велігіна була нагороджена дипломом за найкращу стендову доповідь “Synthesis, characterization, and in vitro anticancer evaluation of 7-piperazin-substituted [1,3]oxazolo[4,5-d]pyrimidines“.](https://bpci.kiev.ua/wp-content/uploads/2025/04/image08.jpg)





Employees of Department No. 2 near the gazebo at the celebration of Chemist's Day on May 23, 2025.
V.P. Kukhar Institute
of Bioorganic Chemistry and Petrochemistry
NAS of Ukraine
© 2026 IBOPC NAS of Ukraine